
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
II. Learn the words and special terms from the list.
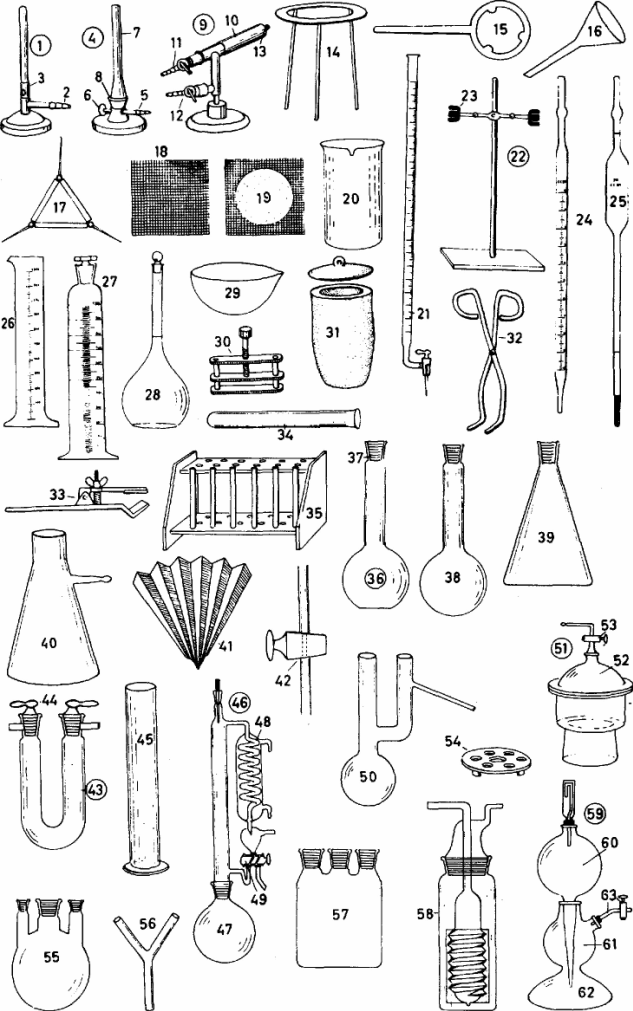
I. Match the word with its definition.
| 1) funnel 2) beaker 3) microscope 4) slides 5) electric balance 6) tongs 7) mortar 8) pestle 9) tripod 10) rubber tubing ll) gas tap 12) matches 13) measuring cylinder 14) test tube 15) test tube rack 16) pipette 17) conical flask 18 ) bung/stopper 19) 1ab coat 20) chemical 21) chemical reaction 22) chemist 23) chemistry | a) a tool that consists of two movable bars joined at one end, used to pick up an object b) a scientific instrument that makes extremely small things look larger c) a short stick with a heavy round end d) the science that is concerned with studying the structure of substances and the way they change e) a round piece of rubber or wood used to close the top of a container f) a round pipe made of rubber for liquids to go through g) a substance used in chemistry or produced by chemistry h) a tube used for pouring liquids or powders into a container with a narrow opening i) an electric instrument for weighing things j) a natural process in which the atoms of chemicals mix and arrange themselves differently to form new substances k) a glass container used for measuring liquid l) a thing glass tube for sucking up exact amounts of liquid, used especially in chemistry m) a small glass container that is shaped like a tube and is used in chemistry n) a piece of clothing that is worn over your clothes in laboratory to protect them o) a scientist who has a special knowledge in chemistry p) a glass cup with straight sides that is used in hemistry for measuring and heating liquids q) small pieces of thing glass used for holding something when you look at it under a microscope r) a hard bowl in which substances are crushed into powder or very small pieces with a pestle s) a special type of bottle mat you use to keep liquids t) a special shelf for tubes u) a support with three legs, used for a camera, telescope etc. v) small wooden sticks, used, to light a tire w) a piece of equipment for controlling the flow of gas from a pipe or container |
V. Describe the functions of each piece of equipment.
VI. Read and translate the text
LABORATORY
A) All the laboratories of inorganic chemistry are almost alike. These are large rooms where both students and research-workers carry out their experimental work. Modern laboratories of inorganic as well as organic and analytical chemistry are provided with gas and running water. Every laboratory is to be provided with a ventilating hood for the escape of both harmful and unpleasant vapours and odours. Every laboratory has to be lit up very well.
There are many laboratory benches with a great number of drawers in every laboratory. Different apparatus devices as well as materials are to be kept in them. Besides we can see many shelves and cases for containers with chemicals.
On every laboratory bench one can see test-tubes, flasks, beakers, funnels,
Evaporating dishes, weighing bottles. All this glassware should be kept in good order.
Various burners serve for producing flames. Bunsen burner is to be mentioned
among them. Different crucibles are to be employed when heating of solution and igniting of materials are to be carried out. Crucibles are usually made of quartz, porcelain and iron. In addition to these crucibles, there are platinum crucibles in some laboratories, but they are used very seldom.
B) Every laboratory should be equipped with different kinds of apparatus. Everything
in the laboratory is to have its definite place. Experiments in the Laboratory Many experiments can be carried out in the laboratory of inorganic chemistry. Thus, if we want to obtain hydrogen chloride (HCL) which is often referred to as a hydrochloric acid gas, it is necessary to pour some sulphuric acid through a tube over the crystal of sodium chloride, in a flask. The fiask is to be heated. On warming the fiask, the hydrogen chloride is expelled as a colourless gas with a suffocating odour. It produces heavy clouds of white fumes when it comes in contact with the moist air of the room.
It is soluble and it cannot be collected over water as are oxygen and hydrogen. It is
much heavier than the air and may be passed through a glass tube to the bottle. If we
dissolve some of the gas in water, the solution has a sour taste, reddens blue litmus, reacts with zinc, etc.: it is hydrochloric acid. When all the sodium chloride originally present in the flask has been transformed, the reaction is complete. The flask then contains a salt called sodium acid sulphate (NaHSO4) together, with unchanged excess of sulphuric acid.
Nitric acid may be prepared by the reaction of concentrated sulphuric acid with
sodium nitrate. In the laboratory method, a mixture of sodium nitrate and
concentrated sulphuric acid is heated in a glass retort. Nitric acid is boiled out of the
mixture and is condensed: NaNO3 + H2SO4 = HNO3+ NaHSO4
Answer the following questions:
1. What do we call a laboratory'?
2. In what laboratories can the students carry out their experiments?
3. What is every laboratory provided with?
4. Why is every laboratory provided with a ventilating hood?
5. What can you see on the shelves'?
6. What glassware is there on every laboratory' bench?
7. What are burners used for'?
8. What are crucibles used for'?
9. What are crucibles made of?
10. What is it necessary to do if we want to obtain hydrogen chloride'? (describe the experiment)
11. How can nitric acid be prepared in the laboratory?
(Возврат Урок4)
Date: 2015-12-17; view: 4474
| <== previous page | | | next page ==> |
| Завершите утверждение согласно содержанию текста. | | | AND COMPOUNDS OF CARBON (Назад на главную) |