CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Fluorescent Minerals
Learn about the minerals and rocks that "glow" under ultraviolet light

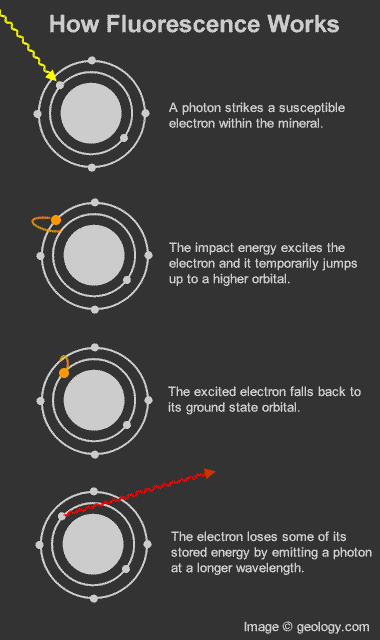
| One of the most spectacular museum exhibits is a dark room filled with fluorescent rocks and minerals that are illuminated with ultraviolet light. They glow with an amazing array of vibrant colors - in sharp contrast to the color of the rocks under conditions of normal illumination. The ultraviolet light activates these minerals and causes them to temporarily emit visible light of various colors. This light emission is known as "fluorescence". The wonderful photograph above shows a collection of fluorescent minerals. It was created by Dr. Hannes Grobe and is part of the Wikimedia Commons collection. The photo is used here under a Creative Commons license. What is a Fluorescent Mineral? All minerals have the ability to reflect light. That is what makes them visible to the human eye. A few minerals have an interesting physical property known as "fluorescence". These minerals have the ability to temporarily absorb a small amount of light and an instant later release a small amount of light of a different wavelength. This change in wavelength causes a temporary color change of the mineral in the eye of a human observer. The color change of fluorescent minerals is most spectacular when they are illuminated in darkness by ultraviolet light (which is not visible to humans) and they release visible light. The photograph above is an example of this phenomenon. Fluorescence in More Detail Fluorescence in minerals occurs when a specimen is illuminated with specific wavelengths of light. Ultraviolet light, x-rays and cathode rays are the typical types of light that trigger fluorescence. These types of light have the ability to excite susceptible electrons within the atomic structure of the mineral. These excited electrons temporarily jump up to a higher orbital within the mineral's atomic structure. When those electrons fall back down to their original orbital a small amount of energy is released in the form of light. This release of light is known as fluorescence. [1] The wavelength of light released from a fluorescent mineral is often distinctly different from the wavelength of the incident light. This produces a visible change in the color of the mineral. This "glow" continues as long as the mineral is illuminated with light of the proper wavelength. |
|
Date: 2014-12-29; view: 1526
| <== previous page | | | next page ==> |
| The Sliding Rocks of Racetrack Playa | | | The Next Energy "Game Changer"? |