
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Thermoluminescence dating
A totally distinct form of thermal study is thermoluminescence dating. This is a method used to try to establish the age of archeologi-cal specimens. The method is based upon the idea that some materials, such as rocks and minerals, emit visible light when heated. The amount of light emitted is believed to relate to the amount of exposure to natural radioactivity. This exposure is built up over an extended period of time. As a result, the greater the amount of light released, the older are the rocks. The age can be estimated by comparing the light intensity with the temperature. The same principle is now employed in personnel badges used for monitoring exposure to radioactivity in laboratories.
A modern instrumentfor
carrying out differential thermal analysis has a computerized temperature controller. It records the release or absorption of heat energy by a sample while that sample undergoes chemical changes or changes in its physical makeup.
| 4H,0 |
Thermal gravimetric graph
200 400 600 800 1,000 1,200 °C
Temperature

|
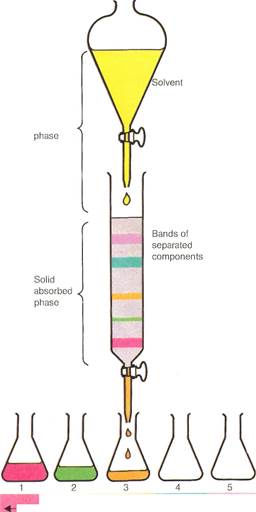
In column chromatography,a mixed solution is poured into the column. A solvent is then trickled through (moving solvent phase) to separate the mixture into bands (solid absorbed phase). The various fractions can then be washed through one at a time using a series of other solvents.
Chromatography
The basic principle underlying all methods of chromatography is simple. A substance in either liquid or gaseous solution (known as the mobile phase) passes through a stationary phase. The stationary phase is a packed column or the surface of a solid material. The mobile phase slows down in its progress due to its interaction with the stationary phase.
| Moving solvent |
| -—'-"—■ Fractions |
In effect, the stationary phase acts as a molecular obstacle course. The molecules of one chemical species move faster or slower than those of another. This depends upon their chemical nature and molecular sizes. What actually happens with the materials in solution is that the mobile and stationary phases compete for them. The greater the attraction to the stationary phase, the slower the compound in solution moves. Conversely, the greater the attraction to the mobile phase, the faster the compound moves. Because of this different rate of movement, chemicals can be separated from each other. They can then be identified by the rates at which they move under specified conditions. Chromatographic methods are very valuable. The three analytical objectives of separation, identification, and quantification (counting the number of molecules or ele-
ments) can frequently be achieved at the same time.
Date: 2015-12-11; view: 3463
| <== previous page | | | next page ==> |
| Radiochemical analysis | | | Computerized control systems |