
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Amino acid degradation and the urea cycle
As well as being oxidized occasionally to produce energy, amino acids are also broken down during the normal turnover of protein in the tissues. If the amino acid unit is not needed in forming new protein, it is oxidized. Excess amounts of amino acids absorbed in food are also oxidized. The amino group removed at the start of amino acid oxidation has to be expelled from the body. This is because it contains ammonia, a very poisonous substance. An intermediate in the citric acid cycle accepts the amino group and then releases it as an ammonium ion. Another intermediate

also readily accepts amino groups, forming aspartate. Aspartate plays an important part in nitrogen elimination. Nitrogen is the main constituent of ammonia. So it has to be converted to a form in which it can be passed out of the body in small volumes of water. This compound is urea. The urea cycle pathway in which it is made occurs in most land animals. Fishes and some other aquatic animals excrete ammonia directly. This is because they have plenty of water available and are not likely to dehydrate. Before entering the cycle, the ammonia reacts with bicarbonate and ATP to form carbamoyl phosphate. This then reacts with ornithine to produce citrulline. This compound combines with aspartic acid to form ar-ginosuccinic acid. This acid, in the next stage, breaks down into the by-product fumaric acid and arginine. Finally, arginine breaks down to ornithine (for reuse in the cycle) and urea.
Photosynthesis
Photosynthesis is the means by which plants, and ultimately animals, derive the energy they need for their metabolic processes. Light energy is used to synthesize (produce) organic molecules from water and carbon dioxide. In some ways, it is therefore a reverse of the process of respiration. It takes place in organelles (minute specialized parts of a cell) of the plant called chloroplasts. Chloroplasts have structural and functional analogies to mitochondria. Chloroplasts are thought to have originated from blue-green algae in the sea, mitochondria from aerobic bacteria on land. Mitochondria, however, are found in all organisms. Chloroplasts occur only in green algae and higher plants. Certain bacteria and the blue-green algae also photosynthesize. But the process takes place in structures that are simpler than chloroplasts. Enormous amounts of light energy are absorbed by plants each year during photosynthesis. Much of this occurs in marine algae.
Pi
(b). ph
Bi
te
 Light of wavelength 680nm
Light of wavelength 680nm
Light of wavelength 700nm
 | |||||
 | |||||
 | |||||
|
|
| M PQH2 |
Electrons
T
Photosynthetic pigment II
T
| c |
Hydrogen
Hydrogen carrier system
Electrons
I
Photosynthetic pigment
|
| Photocenter I |
| NADP |
Photocenter II
| c |
Water
c
I
Electrons
Oxygen
Hydrogen ions
~7\
ADP ^p
T
NADPH„
NADP + electrons
Biochemistry: Biochemical energy 121
The light reaction
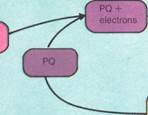
Photosynthesis occurs in two stages. The first stage needs the presence of light, whereas the second can take place in darkness. The light reaction is dependent on the green plant pigment chlorophyll. This is a porphyrin compound containing magnesium. It is related to hemoglobin. There are several similar pigments in chlorophyll. Only the blue-green chlorophyll (a) is common to all plants. Most plants also contain yellow-green chlorophyll (b), xanthophyll (yellow), carotene (orange), and pheophytin. Pheophytin is a gray pigment that is probably a breakdown product of chlorophyll. Each pigment absorbs light of a slightly different wavelength. The purpose of the light reaction is to release hydrogen from water. This reduces carbon dioxide to carbohydrates. It takes place in structures called thylakoids, which resemble stacked plates. All the later reactions occur in the liquid, or stroma, of the chloroplast.
There are two types of light centers in thylakoid membranes. These centers are called photosystems I and II. Absorption of light of a particular wavelength by pigment at center II excites the molecule in center I. Electrons are released. This process reduces (combines with hydrogen) a benzene compound called quinone on the other side of the membrane. The electrons are replaced from water molecules. This process releases oxygen gas and hydrogen ions. Absorption of light at another wavelength by chlorophyll at center I also causes electron release. This time, a phosphate (NADP) is reduced. Both quinone and the phosphate are hydrogen carriers. Their reduction by electrons produced in the light reactions also requires the uptake of hydrogen ions. A compound of quinone and hydrogen is then reoxidized by a sequence of carriers (including cytochromes). Electrons are eventually transferred to replace those ejected from center I. Hydrogen ions are released inside the
thylakoid. The net effect of the two photosystems is that the phosphate is reduced; water is split; oxygen is released; and hydrogen ions are separated across the membrane. These ions then return. A special enzyme is used to conserve the energy released by this process as ATP. This is the same principle that underlies ATP production by mitochondria.
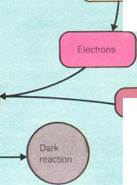
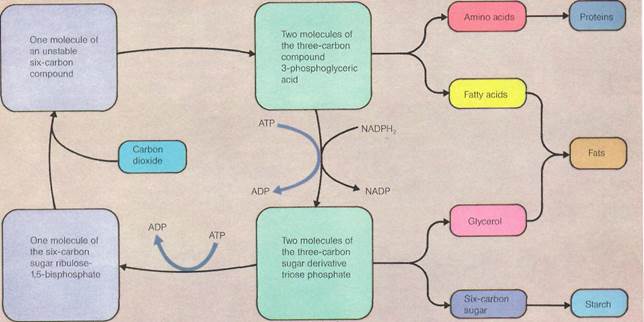
The dark reaction
The dark reaction begins with the combination of a carbon dioxide molecule with a five-carbon sugar. This splits to form two further molecules of a glyceric acid. The acid is then reduced by the hydrogen carried by a phosphate compound. This forms a three-carbon triose phosphate. This can then be used to build up the hexose sugars that are later stored as starch.
But not all the triose phosphate goes to make sugars. Some has to be used in reforming a type of phosphate. By doing so, carbon dioxide continues to be taken up by a pathway known as the Calvin cycle. Some of the steps of this cycle—including the reduction of phos-phoglyceric acid—require ATP. This is available as a result of the light reactions. Studies with radioactive carbon have shown that phos-phoglyceric acid is also vital in building up amino acids and fats.
The dark reaction/below) is the second part of photosynthesis. The light reactions have generated energy (in the form of ATP) and NADPH2 (a reduced phosphate). These are now used to build up complex organic molecules such as starch, fats, and proteins. The various stages of the dark reaction are explained in detail in the main text.
|
 |

|
Date: 2015-12-11; view: 1883
| <== previous page | | | next page ==> |
| Fatty acid oxidation | | | The metamorphic stages |