
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Heterocyclic nitrogen compounds
Heterocyclic compounds are ring structures that include at least one atom other than carbon in the ring. Aromatic heterocyclic compounds of nitrogen contain a nitrogen atom in the ring. These compounds are important as biochemical intermediates, helping in the formation of complex compounds. In some respects, their chemical properties are similar to benzene derivatives. For example, they may display aromatic character. This means that some of the electrons of heterocyclic nitrogen compounds may be diffused around the ring structure, being shared by all the atoms in the ring. One of the most common of these compounds is pyridine, pyridine is a six-membered ring with one carbon atom substituted by a nitrogen atom. It reacts as amines do with acids to give salts. It can be extracted from coal tar. Its derivatives are important in nature (for example, nicotinic acid, a B-complex vitamin).
Quinine is a natural derivative of pyridine. It occurs in the bark of the cinchona tree. It is used in making medicines for malaria and also as a flavoring for tonic water, sometimes called quinine water.
Pyrrole is afive-membered heterocycle with four carbons and one nitrogen in the ring. Considered to be aromatic, it can be isolated from coal tar or made industrially from ammonia and furan (a colorless liquid prepared from an aldehyde). Other pyrroles can also be prepared easily by a variety of synthetic (artificial) methods.
An important derivative of pyrrole is indole. Indole is formed when a benzene ring is fused to a pyrrole ring. Indole crystals are colorless with a pleasant odor and can be used in perfumes. Indole's derivatives are widespread in nature. Many alkaloids and hallucinogens (substances that produce hallucinations) are derived from tryptophan, an amino-acid derivative of indole.
Although few pyrroles occur in nature, the
porphyrins are a class of naturally occurring pigments that can be said to have derived from pyrrole. They are not actually pyrrole derivatives, but a separate class of highly-colored stable aromatics. The porphyrin system is of great importance because it forms what are termed chelate compounds. These are compounds that hold on to metal ions (electrically charged atoms). The ions are held between four nitrogens. This is the basis for the red blood pigment hemoglobin and the green leaf pigment chlorophyll.
Human blood, as well as the blood of all vertebrate animals (animals having spines), is able to carry oxygen because of hemoglobin. The key is a six-coordinate iron atom, held on four sides by the nitrogens (the porphyrin complex). This porphyrin complex is the heme in hemoglobin. Underneath, the iron is held by a nitrogen molecule from the protein giobin. This protein is the giobin in hemoglobin. The sixth molecule is oxygen, which is carried from the lungs by the hemoglobin to the various cells of the body. Chlorophyll, the green pigment in plants, is essential in photosynthesis. This is the process by which plant cells make carbohydrates from carbon dioxide and water in the presence of chlorophyll and light.
Aromatic nitrogen compoundsrange from aniline (amino-benzene) to heterocyclic compounds. In these compounds, a nitrogen atom has a place in one of the rings (pyridine to indole).
Nylon 6/6 is a polyamide
polymer.It is built up by the formation of an amide linking an acid and an amine, with the elimination of water (see diagram below). The reactants are heated to a temperature of 518" F. (270° C) under a pressure of 10 atmospheres to make the reaction take place.

|
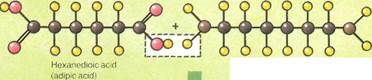
|

|
| 1,6-diaminohexane (hexamethylenediamine) |
Date: 2015-12-11; view: 1577
| <== previous page | | | next page ==> |
| Structure and properties of amines | | | Nitrogen-oxygen compounds (oximes) |