
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Reactions of alkanes
The name paraffin, as the alkanes were originally called, derives from the Latin parum af-

Liquefied alkanesare a
convenient form of portable fuel supply. Butane, for example, is used in cigarette lighters. Camping stoves and lamps, and even automobiles and trucks can also be fueled by liquid alkanes. Such alkanes are called liquid petroleum gas (LPC) in automotive applications.
70 Organic chemistry: Saturated aliphatic hydrocarbons
|
| Molecular model of "chair form of cyclohexane |
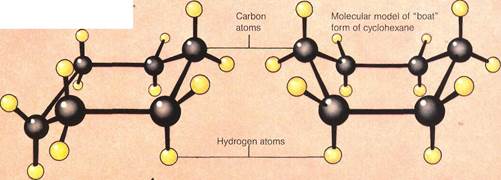
The first four alicyclic hydrocarbonsare illustrated in the diagram (top right!. They resemble both straight- and branched-chain alkanes in consisting of only carbon and hydrogen atoms. But their carbons are linked in a ring structure. This means that alicyclic hydrocarbons have a different general formula from that of their chain-structure analogues: C„H2„ (where n is the number of carbon atoms). The three-dimensional shapes of alicyclic hydrocarbons are often depicted as flat rings. In fact, the rings are puckered in all except cyclopropane. This puckering is exemplified well by cyclohexane. It can exist in several puckered conformations, two of which are illustrated (lower right). Any one cyclohexane molecule can be flexed into different conformations. These vary from the "chair" form through various intermediates to the "boat" form, and back again. But not all of these forms are equally stable. The chair form is the stablest. At any given moment about 99.9 per cent of cyclohexane molecules are in this form.
| Cyclopropane (C3H6) | Cyclobutane (C„HB) | Cyclopentane (G5H10) | Cyclohexane (C6HB) |
| Structural formula | Structural formula | Structural formula | Structural formula H H |
| H H \ / C M~"~C--------- C""M / \ H H | H H H—C--------- C—H H H | H H \/ H H | I I H J\ HH H |
| Representation of | Representation of | Representation of | Representation of |
| cyclopropane molecule A | cyclobutane molecule | cyclopentane molecule o | cyclohexane molecule |
Cyclohexane molecules twist into different forms, such as the "chair" form (above left, with its representation left) and the "boat" form (above right, representation right)
 finis. This means little affinity, or little attraction, to other substances. In chemical terms, the hydrocarbons are relatively unreactive. All, however, undergo oxidation. They combine easily with oxygen. If they did not, they would not have become important in applications that depend on their combustion (ability to burn).
finis. This means little affinity, or little attraction, to other substances. In chemical terms, the hydrocarbons are relatively unreactive. All, however, undergo oxidation. They combine easily with oxygen. If they did not, they would not have become important in applications that depend on their combustion (ability to burn).
They also undergo other types of reactions. These include halogenation (combining with one of the halogen elements: fluorine, chlorine, bromine, or iodine) and nitration (in which a nitrogen-oxygen group replaces a hydrogen atom). The conditions required to sustain these reactions are often severe.
Under extreme conditions, hydrocarbons will isomerize. This means that they break down partially and then recombine into different hydrocarbon isomers. Such isomerization is at the basis of the process used to increase the octane count of gasoline. The octane number is based on the combustion (burning) properties of normal octane, which is assigned a value of 100. Most gasoline used today has an octane number between 91 and 93.
As the molecular weight of hydrocarbons increases, they become larger and heavier and change from gases to liquids to solids at room
temperature. Petroleum jelly is a soft, solid hydrocarbon mixture obtained from crude oil. It is used as a barrier ointment to protect wounds and sores. Higher molecular weight hydrocarbons form hard waxes. These can be used as protective coatings for floors and furniture or as slow-burning materials—in candles, for example.
Date: 2015-12-11; view: 3596
| <== previous page | | | next page ==> |
| Production and uses | | | The nylon-making plant |