
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Complex inorganic compounds
| Inorganic compounds are substances that contain no organic matter, being neither animal nor vegetable. In chemical terms, inorganic compounds do not contain carbon-to-carbon bonds. For example, carbon dioxide contains carbon, but it is not bonded (attached) to another carbon atom. Carbon dioxide is an inorganic compound. However, compounds containing carbon are usually organic. (Compounds are discussed in the articles that begin with "Organic chemistry.") Many inorganic compounds are not simple two-element substances like carbon dioxide or sodium chloride. Rather, they consist of a number of elements bonded together in a complicated structure. Compounds in which a central atom is surrounded by other atoms, |
ions (electrically charged atoms), or small molecules are known as complexes or coordination compounds.
Complexes can be electrically neutral molecules or electrically charged ions—complex ions. Complexes usually have from 2 to 9 atoms, ions, or groups surrounding a central atom or ion. Some of the many hundreds of thousands of complexes and complex ions have important applications. These range from helping to carry oxygen from the lungs and through the blood of animals to developing images on photographic films.
Ligands

|
| o |
| Straight |
| Bent |
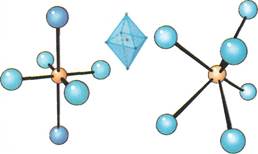
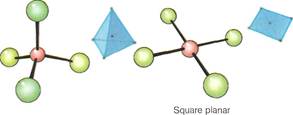 The atoms, ions, or small molecules surrounding the central atom or ion of a complex are known as ligands. The number of them is called the coordination number. Such numbers can run from 2 to 9 and, in some cases, beyond. But the most important complexes in chemistry are those with coordination numbers 2,4, or 6. For example, most metal ions dissolved in water form complex ions with a coordination number of 6. This means that there are 6 ligands surrounding the central atom.
The atoms, ions, or small molecules surrounding the central atom or ion of a complex are known as ligands. The number of them is called the coordination number. Such numbers can run from 2 to 9 and, in some cases, beyond. But the most important complexes in chemistry are those with coordination numbers 2,4, or 6. For example, most metal ions dissolved in water form complex ions with a coordination number of 6. This means that there are 6 ligands surrounding the central atom.
| Tetrahedral |
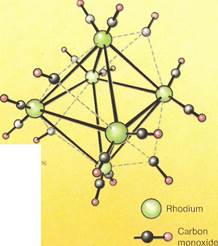
 The formation of complexes is one of the most outstanding properties of metal ions. Observing the changes in their properties has given chemists a valuable insight into the metals themselves and the nature of the chemical bond. For example, complexes involving the metals of the first transition series and ammonia have been extensively studied. These are the metals from scandium to zinc, atomic numbers 21 to 30. Some of these metals also form a wide range of complexes and complex ions with carbonyl (a compound of carbon and oxygen) groups as ligands. The carbonyls have special bonding characteristics.
The formation of complexes is one of the most outstanding properties of metal ions. Observing the changes in their properties has given chemists a valuable insight into the metals themselves and the nature of the chemical bond. For example, complexes involving the metals of the first transition series and ammonia have been extensively studied. These are the metals from scandium to zinc, atomic numbers 21 to 30. Some of these metals also form a wide range of complexes and complex ions with carbonyl (a compound of carbon and oxygen) groups as ligands. The carbonyls have special bonding characteristics.
| Octahedral |
| Triangular prismatic |
Any atom, ion, or molecule with two extra electrons—known as a lone pair—can donate this pair to a central atom. This atom, ion, or I molecule with the lone pair then becomes a li-gand of a complex. Ligands that bond through
Major groups of elements: Complex inorganic compounds 65

|
|

|
| Rh.(CO) |
-o—o
Carbon monoxide
one lone pair are known as monodentates. However, many molecules have more than one atom with a lone pair and can function as bidentate or polydentate ligands, being able to bond through two or more pairs. For example, ammonia is a monodentate ligand because it possesses only one lone pair. The compound 1,2-diaminoethane is a bidentate ligand. This means that it can form two bonds. Polydentate ligands are able to form bonds through three or more pairs.
The carbonyl group (consisting of compounds of carbon and oxygen) also acts as a monodentate ligand, like ammonia, but in a special way. The carbon of the carbonyl group is a poor donor atom. This means that the carbonyl would usually not be expected to act as a ligand. But as a result of its bonding with oxygen, it is able to draw electrons from the central (metal) atom. It also donates a pair of electrons to the metal simultaneously. This gives rise to a so-called synergic bond, which is a very stable bond.
to describe the bonding in complexes, a number of theories have been put forward. These include the valence-bond theory, the crystal field theory, the ligand field theory, and the molecular orbital theory. They are complex theories that try to describe the shapes, colors, and magnetic properties of the complexes.
Shapes of complexes
The shapes of complexes and complex ions has been the subject of much study. In complexes with the most common coordination numbers—2, 4, and 6—eight different spatial arrangements of atoms, ions, and molecules are found. Complexes with a coordination number of 2 can be straight or bent. Complexes with coordination number 4 usually take on a tetrahedral arrangement, having 4 sides. Sometimes, a flat square of atoms or groups is found if all the ligands are the same. Complex compounds with coordination number 6 take on an octahedral shape, having eight sides. These may be distorted. A type of triangular shape also occurs, but less frequently.
When a complex has two or more different ligands attached to the central atom, it may have more than one structural formula. The two different structural arrangements of the same complex are called stereoisomers. When the stereoisomers are mirror images of each other, they are known as optical isomers.
Date: 2015-12-11; view: 1845
| <== previous page | | | next page ==> |
| Interstitial and substitutional alloys | | | Polynuclear complexes |