
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Colorful neon signs are
used for advertising throughout the world.
Welding of aluminumis
carried out under a "blanket" of argon gas. The inert argon does not react with the hot metal, nor does it allow oxygen in the air to oxidize it.
Fact entries
Heliumwas discovered in the mineral clevite in 1895 by the Scottish chemist William Ramsay (1852-1916). It was named after the Greek helios, meaning sun, because it was first detected in the sun's spectrum. At. no. 2; at. mass 4.0026; m.p. -272.2" C; b.p. -268.9° C.
Neonwas also discovered by Ramsay, with Morris Travers (1872-1961), in 1898 in impure argon from air. Its name derives from the Greek neos, meaning new. At. no. 10; at. mass 20.179; m.p. -248.67° C; b.p. - 246.048° C.
Argonwas isolated from air in 1894 by Ramsay and Lord Rayleigh (1842-1919). Its name comes from the Greek argos, meaning inactive. At. no. 18; at. mass 39.948; m.p. -189.2° C; b.p. -185.7° C.
Kryptonwas discovered by Ramsay and Travers in 1898.
It is named after the Greek kryptos, meaning hidden. At. no. 36; at. mass 83.80; m.p. -156.6° C; b.p. 152.3° C.
Xenon,from the Greek word for strange, was also discovered by Ramsay and Travers in 1898. At. no. 54; at. mass 131.29; m.p. -111.9°C; b.p. -107.1° C.
Radonwas discovered by the German chemist Frie-drichDorn (1848-1916) in 1900. It was later isolated by Ramsay. It is formed by the radioactive decay of radium, after which it is named. It is itself radioactive. At. no. 86; at. mass (the most stable isotope) 222; m.p. -71° C; b.p. -61.8° C.

| Be | ||
| 9.0128 | ||
| Mg | ||
| 24.305 | 3B | 4B |
| Ca | Sc | Ti |
| ? 40.08 | 44.9559 | 47.88 |
| Sr | Y | Zr |
| 87.62 | 88.9059 | 91.224 |
| Ba | La | Hf |
| 137.33 | 138.906 | 178.49 |
| Ra | Ac | |
| 226.025 | 227.028 |
The scandium group
makes up Croup 3B of the periodic table. It includes the lanthanides (elements 57 to 71) and the actinides (elements 89 to 103).
Many phosphorscontain lanthanide compounds. These phosphors produce the colors on television screens and on VDU displays for computer graphics.
The scandium group and the lanthanides
Scandium (Sc), yttrium (Y), lanthanum (La), and actinium (Ac) are the four elements that form Group 3B of the periodic table. However, lanthanum and actinium have their own series of related elements, known as the lanthanides and actinides. These form separate groups within the periodic classification. The lanthanides are included in this article. The actinides are the subject of the next one.
In some respects, the Group 3B elements resemble those of Group 3A: boron, aluminum, gallium, indium, and thallium. The resemblance is particularly strong with scandium, whose chemistry is much like that of aluminum. Scandium, yttrium, and lanthanum oxidize (combine with oxygen) readily when exposed to air. These elements also react with water to release hydrogen.
Scandium
Scandium is a soft, silvery-white metal. It is quite common, there being almost as much of the element in the earth's crust as there is of arsenic and almost twice as much as there is

of boron. There are few rich mineral sources of scandium. It usually occurs mixed with other lanthanides in the minerals thortveitite and wiikite. Sometimes, it is found in tin and tungsten ores. In all, it is found in over 800 minerals, although only in very small amounts. Scandium is not separated easily from other elements. It is separated using a special and difficult process involving an exchange of ions (electrically-charged atoms) of the element while in solution. Scandium is employed mostly in high-intensity lights. Astronomers have also found that some stars contain much scandium.
Yttrium
Yttrium is a heavy, silvery-white metal that resembles lanthanum and the lanthanides. It is found in nearly all minerals that contain the rare earths. It is obtained commercially from monazite sand. Yttrium has various uses, especially in the electronics industry. A compound called yttrium oxide is used in color television screens as the basis of the color red. The same compound is also used to make a kind of crystal called a garnet. One type of garnet is used as a filter in radar. Another type serves as an imitation diamond. Yttrium is also used in lasers and in manufacturing certain ceramics, chemicals, and glass. It finds use as a catalyst and in the making of various alloys.
Lanthanum
Lanthanum is a soft and pliable silver-white metal. It is found together with the lanthanides in monazite, bastnasite, and other minerals. It is also produced during nuclear fission (the splitting of atoms), which involves uranium, thorium, and plutonium. Lanthanum finds use in different alloys, especially in making lighter flints. It is also used as a catalyst and in the glass industry, in which it is employed in camera lenses and carbon-arc searchlights.
The rare earths
The lanthanides are also known as the rare earth elements because they were found at first only rarely, and then only as oxides (com-

|
Monaziteis a phosphate mineral that is the principal source of cerium and other lanthanides, and of thorium. It occurs in beach sands along the southwestern coast of Pakistan.
Major groups of elements: The scandium group and lanthanides 59

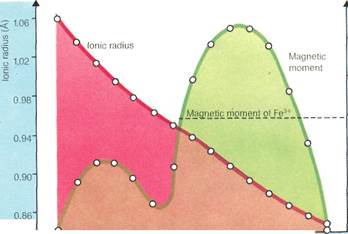 bined with oxygen). These oxides resemble calcium, magnesium, and aluminum oxides, which are sometimes known as the common earths. By modern standards, however, they are not rare. Some of them are even produced on a large scale. Even the scarcest—thulium-is as common as bismuth and occurs in more abundance than arsenic, mercury, cadmium, or selenium.
bined with oxygen). These oxides resemble calcium, magnesium, and aluminum oxides, which are sometimes known as the common earths. By modern standards, however, they are not rare. Some of them are even produced on a large scale. Even the scarcest—thulium-is as common as bismuth and occurs in more abundance than arsenic, mercury, cadmium, or selenium.
Apart from promethium, the lanthanides always occur together—most commonly in the mineral monazite, a dense, dark sand that contains mixtures of lanthanide phosphates. These are mixtures of a variety of the rare earths combined with phosphorus. The lanthanides are also found in combination with other non-metallic elements in the form of carbonates (with carbon), silicates (with silicon), and fluorides (with fluorine). Lanthanum, cerium, praseodymium, and neodymium make up to 90 per cent of all mineral deposits of lanthanides. Yttrium and the heavier elements of the group make up the rest. Promethium does not occur naturally. Several artificial isotopes have been made, sometimes as a product of nuclear fission. But these are all short-lived.
Until the end of World War II, the extraction of rare earth elements was a long, complicated, and costly process. There are two processes in use today, the ion-exchange and the solvent extraction processes. These chemical processes have made possible a rapid separation that gives highly pure, low-cost rare earths.
True rare earths, after separation from their oxides, are silver-colored metals. These separated rare earths are used in lasers, magnets, lamps, television screens, and some kinds of X-ray equipment. Unseparated rare earths help make metals, such as aluminum and magnesium, stronger. Another type of rare earth alloy (called misch metal) is combined with iron to make flints for cigarette lighters. Rare earth elements are also used in motion-picture projectors and in the production of various petroleum and synthetic products.
-i—i—i—i—i—i—i—i—i—r-
La3' Ce31' Pr3' Na- Pm3' Sm3* Eu3' Gd3- IV Dy3' Ho-" Er34 Tm3* Yb3' Lu3*
Lanthanide ions
The lanthanide contractionrefers to the steady decrease in the size of the lanthanide ions along the series from lanthanum to lu-tetium (rather than the expected increase).
| Element | Symbol | Atomic number | Atomic weight | Discovery date | Discoverer |
| Cerium | Ce | 140.12 | W. von Hisinger, Jons Berzelius, and Martin Klaproth | ||
| Praseodymium | Pr | 140.907 | Carl von Welsbach | ||
| Neodymium | Nd | 144.24 | Carl von Welsbach | ||
| Promethium | Pm | 145.00 | J.A. Marinsky, L.E. Glendenin, and CD. Coryell | ||
| Samarium | Sm | 150.35 | Lecoq de Boisbaudran | ||
| Europium | Eu | 151.96 | Eugene Demarcay | ||
| Gadolinium | Gd | 157.25 | J. de Marignac | ||
| Terbium | Tb | 158.924 | Carl Mosander | ||
| Dysprosium | Dy | 162.50 | Lecoq de Boisbaudran | ||
| Holmium | Ho | 164.930 | j.L Soret | ||
| Erbium | Er | 167.26 | Carl Mosander | ||
| Thulium | Tm | 168.934 | PerTheodor Cleve | ||
| Ytterbium | Yb | 173.04 | J. de Marignac and George Urbain | ||
| Lutetium | Lu | 174.97 | George Urbain | ||
Fact entries
Scandiumwas one of the "missing" elements predicted by Dmitri Mendeleev. It was discovered in 1879 by the Swedish chemist Lars Nilson (1840-1899). He named it after Scandia, the Latin name for Scandinavia. At. no. 21; at. mass 44.9559; m.p. 1539° C; b.p. 2832' C.
Yttriumis one of several elements named after the town of Ytterby in Sweden. It was discovered by the Swedish chemist Carl Gustav Mosander (1797-1858) in 1843 in the mineral yttria. At. no. 39; at. mass 88.9059; m.p. 1522±8° C; b.p. 3338° C.
Lanthanumwas discovered in 1839 as an impurity in cerium by Carl Mosander. Its name derives from the Greek lanthano, which means to be hidden. At. no. 57; at. mass 138.906; m.p. about 920° C; b.p. about 3469° C.
Lanthanides(rare earth elements) are listed in the above table, which gives brief details of the atomic number and weight and the discovery of each of them.
Actiniumis described in the following article.
Date: 2015-12-11; view: 1769
| <== previous page | | | next page ==> |
| Sodium chloride crystals | | | The actinides and beyond |