
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Arsenic, antimony, and bismuth
Arsenic, antimony, and bismuth exist in bright, metallic forms that are stable in air. They are found free in nature or in combination with other elements, usually sulfur. They are most often used to improve the strength and hardness of alloys, which are combinations of metals.
Arsenic is a gray, shiny metalloid. This is I the ordinary, stable form of the element. There are two other forms—yellow arsenic and black
Major groups of elements: Phosphorus to bismuth 49
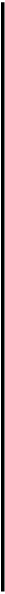
|

|

|
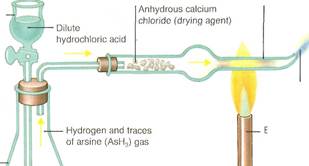
| Metallic mirror of arsenic |
| Funnel |
| Hydrogen burning |
| unsen burner |
| Flask |
| Forensic sample Arsenic-free zinc |
| A Phosphorus match I:' si' i |


arsenic. Occasionally found free in nature, arsenic usually occurs in combination with sulfur, oxygen, or certain metals. Arsenic is a deadly poison. It is used in the manufacture of fungicides, weedkillers, rat poisons, and insecticides. It is also used in certain types of electrical equipment and to increase the strength of certain alloys.
Antimony is a bluish-white, brittle metal. This is its more common pure form. It also exists as black or gray antimony, a black powder. Antimony is most often found, however, in combination with sulfur in a mineral called stibnite. Compounds containing antimony are used in refrigerators, air conditioners, paints, aerosol sprays, and flame-proofing materials. Antimony-lead alloys are used in making metal for printing type, batteries, and electric cables.
Bismuth is a pinkish-white, brittle metal. Although it is found free in nature and in certain ores, such as bismite, it is generally obtained as a by-product in refining tin, lead, copper, silver, and gold ores. Bismuth is most often used to make alloys that melt at low temperatures. These alloys are used to make safety plugs for steam boilers and automatic sprinkler systems. They are also used in making electrical fuses. Bismuth finds uses in foundries and in nuclear reactors. It is also used in some medicines, cosmetics, and certain drugs.
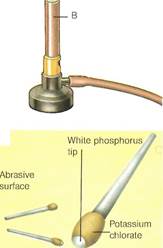
| Abrasive surface |
B Strike-anywhere match
C Safety match

V_
Phosphorus
sulfide
tip
Potassium ""chlorate and sulfur
 Antimony sulfide >-and potassium dichromate
Antimony sulfide >-and potassium dichromate
-Red phosphorus and abrasive powder
Antimony compounds
were known to the ancient Egyptians. They used a sulfide (stibnite) as black eye makeup, a feature reflected in their sculpture (far left!.
In the Marsh test(left), devised as a forensic test in cases of suspected arsenic poisoning, the gas arsine is heated to form a metallic mirror of arsenic.
Phosphorushas long been used in matches. The original phosphorus match (A)— now illegal—had a white phosphorus tip. It was banned because of the danger of phosphorus poisoning to match workers. A strike-anywhere match (B) uses phosphorus sulfide instead. In safety matches (Q, the nonpoisonous red phosphorus is in the striking surface.
The two main allotropes of phosphorus(below) have different molecular structures. White phosphorus (A) exists as tetrahedrally joined groups of four atoms. Red phosphorus (B) consists of chains.
Fact entries
Phosphoruswas first isolated from urine by the German alchemist Hennig Brand in 1669. In 1860, it was discovered independently by the English chemist Robert Boyle. The name comes from the Creek phosphoros, meaning light-bearer. The white form
glows in the dark. At. no. 15; at. mass 30.9738; m.p. (white) 44.1° C; b.p. (white) 280° C; m.p. (red) 600° C (under pressure).
Arsenicwas identified by Albert Magnus about 1250. The Latin word arsenicum means yellow orpiment (a
pigment containing arsenic and sulfur). At. no. 33; at. mass 74.9216; sublimes (passes directly into a vapor without melting) at 613° C
Antimonyoccurs mainly as its sulfide mineral stibnite (Latin stibnum), from which it derives its chemical sym-
bol Sb. It was known to the Creeks and Romans. At. no. 51; at. mass 121.75; m.p. 630.74° C; b.p. 1586.85° C
Bismuthwas known in the ancient world. It was isolated by Caspar Newmann (1683-1737). Its name may derive from the Old German
vissmuth (white matter). At. no. 83; at. mass 208.98; m.p. 271.3° C; b.p. about 1560+5° C.
| 5A | 6A | /A |
| N | F | |
| 14.0067 | 15.9994 | 18.9984 |
| P | s | CI |
| 30.9738 | 32.06 | 35.453 |
| As | Se | Br |
| 74.9216 | 78.96 | 79.904 |
| Sb | Te | I |
| 121.75 | 127.6 | 126.905 |
| Bi | Po | At |
| 208.98 | (209) | (210) |
Oxygenheads Group 6A of the periodic table. It is more reactive than nitrogen, its neighbor to the left in Croup 5A, but less so than fluorine on the right.
Oxygen
Oxygen (O) is a colorless, odorless, and tasteless gas. It is one of the most abundant elements on the earth. It makes up about 23 per cent by weight and 21 per cent by volume of the atmosphere, 89 per cent by weight of water, and almost half the weight of the earth's crust. In air, oxygen is found in molecules that contain two atoms of oxygen. Elsewhere, it is found combined with many other elements as oxides, in various salts, and as an important part of living matter.
In the periodic table, oxygen lies at the head of Croup 6A. By taking up two electrons from other elements, it can acquire a full outer shell of electrons and a stable structure. By doing this, it can combine with almost every other element—both metals and nonmetals. Compounds of the other elements and oxygen are known as oxides. These can be formed in a variety of ways. For example, finely divided iron or carbon burn in pure oxygen to form oxides. Iron left in the air slowly rusts, also to form iron oxide. Such reactions with oxygen are known as oxidation. Most combustion reactions, such as ordinary wood fires, involve oxidation. In these cases, a very fast burning process also gives out large amounts of heat and light. The decay of plant and animal tissue also involves oxidation, but without the rapid release of heat and light.
Date: 2015-12-11; view: 1845
| <== previous page | | | next page ==> |
| Ammonia and hydrazine | | | The role of oxygen in biology |