
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Introduction to Polymer Science and Technology
Polymerisation
The gas flow, consisting of monomer/comonomer, hydrogen, nitrogen (inert carrier gas), and inert condensing agent, provides monomer/comonomer for polymerisation, agitates the bed, and removes the heat of polymerisation. The operating temperature is approximately 90 °C for LLDPE and 100 °C for HDPE and the pressure of approximately 10 bar (1 MPa). The gas with some polymer/catalyst particles rises to the enlarged domed section, referred to as the disengagement zone, where a variation in velocities occurs and entrained particles disengage from the gas, before the gas leaves the reactor, and drop back into the reaction zone. The condensing agent can be monomers and inert liquids (e.g., pentane, isopentane, butane, hexane), and its heat of vaporisation results in cooling in the reactor. The boiling point of the condensable liquid has to be lower than the operating temperature of polymerisation, for effective control of reaction. The gas leaving from the top of the reactor is condensed in the heat exchanger and returned to the reactor in liquid form.
The polymer powder passes to a purge vessel where a deactivating agent (the weight ratio of the deactivating agent to the catalyst is approximately 0.001) kills all catalyst activity, nitrogen strips off traces of monomers from hot powder, and a small amount of steam (a few kg/h) removes triethylaluminum (TEA) and other non-monomer chemicals. Finishing of the polymers includes addition of additives (e.g., heat, UV and perhaps other stabilisers, lubricants, pigments, colorants, etc.), drying, extrusion and pelletising. The polymer may enter the extruder quite hot and therefore may necessitate cooling, rather than heating, along the extruder barrel. The temperature of the cooling water for pelletisation is critical: fast and/or slow cooling/solidification can produce pellets with defects as shown in Figure 2.4. There are other causes / conditions that can generate defected pellets, which are documented by the company Black Clawson and/or Davis Standard in their company publications & technical articles, see their web sites: http://www.er-we-pa.de/home.html, http://www davis-standard.com/

|
| Internal voids |
| Twins/triplets/chains |
Agglomeration/clustering
Figure 2.4Various types of defects in pelletisation: fast cooling resulting in voids and slow cooling in agglomeration of pellets from twins to large clusters (source: http://www.er-we-pa.de/public html/Companv/pubs/EP defects.html)
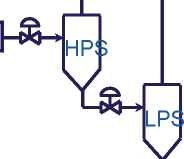
A typical high pressure tubular processfor the production of LDPE is illustrated in Figure2.5. In this process, the ethylene monomer is gradually compressed to a pressure level suitable for the reaction, up to 3000 bar (300 MPa) with a tubular reactor. The free radical polymerization initiates at about 150 °C, using oxygen or an organic peroxide as initiator. The temperature of polymerization reaction can peak to over 300 °C. A mixture of polymer and unreacted monomer is transported from the reaction tube to a separating and recycling part, which includes a high-pressure separator (HPS). The HPS is connected to a
Introduction to Polymer Science and Technology
Polymerisation
low-pressure separator (LPS) for further monomer removal. The resulting molten polymer phase is passed from the LPS to a polymer finishing section for extrusion. The unreacted monomer separated in HPS gets recycled at a pressure similar to that of the outlet of the primary compressor and combines with the monomer containing feed passing from the primary to the secondary compressor. The unreacted monomer from the LPS recycles through the primary compressor.
low pressure recycle

|
| reactor (1-3 kbar) |
| [compressor -LTD" |
 ÑÒÀ
ÑÒÀ
£L
Ethylene

|
high pressure recycle
Secondary hypercompressor
purge
L
lextruder}-»
Compression -» Reaction -» Devolatilization -» Extrusion
Figure 2.5An illustration of typical high pressure LDPE process
Chain transfer agent (ÑÒÀ) is also added to the circuit and conveyed to the reactor. ÑÒÀ is used to reduce the molecular weight (MW) and to narrow the molecular weight distribution without changing the overall rate of conversion of monomer to polymer (using more initiator is another way to decrease MW, but the reaction rate would increase proportionally with a risk of a dangerous situation arising).
Further information on polymerisation mechanisms and processes can be found, amongst many others, in Asua 2007 and Fried 1995. A description of these processes as well as environmental, health and safety guidelines in association with these processes is also presented in a report by the International Finance Corporation 2007.
2.4 Catalysts
The employment of polyolefins enjoys a massive global increase: PE has the worlds largest market closely followed by PP, which is experiencing the highest growth rate in many years. The development of polyolefins into the largest-volume family of commercially important, high-tonnage thermoplastic polymers has been made possible with the advent of coordination catalysts.The coordination catalyst types include Philips catalysts (supported chromium oxide catalyst), Ziegler-Natta and single-site catalysts, e.g., constraint geometry and metallocene.
Date: 2015-12-11; view: 1318
| <== previous page | | | next page ==> |
| Figure 2.2An illustration of the process of emulsion polymerisation | | | Introduction to Polymer Science and Technology Polymerisation |