
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Chapter 13 FIRING
Firing is a heat application process during which a ceramic product undergoes:
a change in volume;
a change in surface aesthetics;
t melting of some of its mineral components with subsequent transformation into glass;
crystal transformations with the formation of new phases;
a drop in permeability;
increases in tensile strength.
13.1 - CHEMICAL AND MINERALOGICAL REACTIONS
Sanitaryware firing is divided into three stages:
Heating;
Holding at maximum temperature;
Cooling.
The changes in temperatures from the start of the firing cycle to its end form the firing curve of a material.
During healing the temperature is raised from ambient temperature to around Iijoo JC, the rate at which the temperature is raised (the thermal gradient) depends on the company's specific production requirements and the changes that take place inside the ceramic. The body consists of minerals, which undergo changes; the main phase-by-phase changes and their respective activation temperatures are described below:
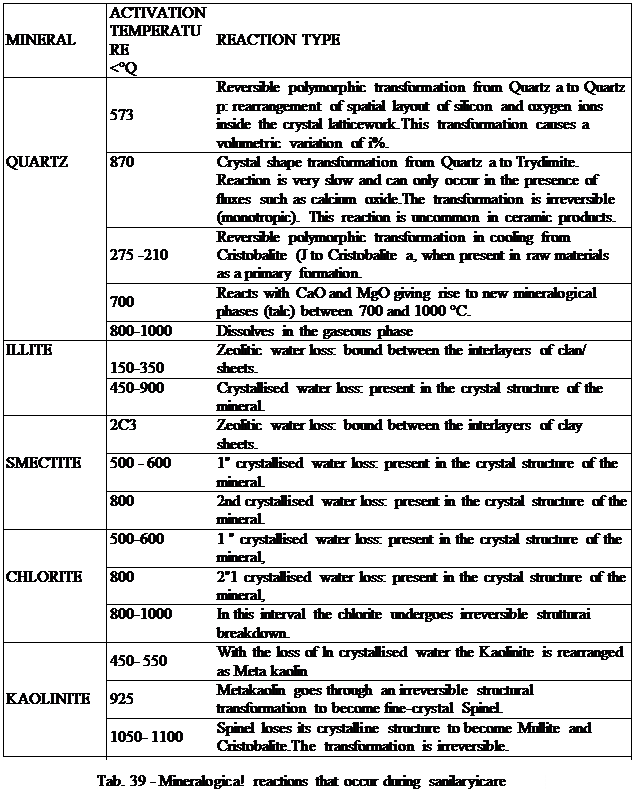
Mineral transformations involve volumetric changes that can compromise the quality of the object depending on their extent and the quantity of the phase contained in the body. Reversible firing transformations occur during both heating and cooling.
During the initial heating gradients the body loses residual hygroscopic water, which should, in any case, have been reduced to less than 0.5% by the drying process. The change in volume caused by water loss is minimal.
With the increase in temperature, in the range between 150 and 300 °C, (see table n° 39), the laminar-structure clay minerals lose their interlayer water, generating very small decreases in volume.
At about .3(X>°C organic substance combustion reactions In gin and these continue until 500 °C. Organic substances ar e concentrated in the clay minerals (by deposition and diagenesis dynamics) and their content, especially where particle size is high, is regulated by sieving during body preparation. Organic substance combustion produces carbon dioxide and water vapour; carbon ions residues might remain in the fine porosity of the body, produced by the evacuation of these substances.
The black core' so typical of tile production is uncommon in sanitaryware. This is because the three-dimensional nature of the pieces obliges the user to engage in firing cycles that allow for the evacuation of reaction products and because the raw materials are usually more refined to prev ent any colouring of the body.
If the body contains Cristobalite, at 210 °C a polymorphic transformation from jhase a to phase B (i.e. reversible) takes place. The expansion coefficients and quantities of the mineral contained in the body are too low to compromise the characteristics of the ceramic product.
Between 450 and 900 °C the clay minerals lose, at different temperatures and in different ways depending on their crystal structure, the crystallised water (bound as hydroxyl ions inside the latticework), producing water vapour. At this stage it is especially important that there bea transformation of the kaolin, which undergoes a structural rearrangement to become metakaolin. The loss of crystallised water causes negative volumetric variations.
At 578 °C the quartz undergoes a reversible polymorphic transformation from its a form to its fj form, giving rise to an increase in volume of 3%, partially offset by the shrinkage induced by water loss. While such contraction is considerable, it does not. at this stage of firing, cause any damage in that it is absorbed by a still-porous body that is less rigid than its fired counterpart.
The transformation involves a drop in density and it can, overall. In* stated that the hotly undergoes general weight loss.
Over 600° and up to 1000 °C there is decarbonation of the glaze carbonates and the release of calcium oxide, magnesium oxide and carbon dioxide.
At !J5<> °C kaolinite, which has already Involile Metakaolin following the loss of 1st crystallised water, is rearranged to form Spinel.
At 950 °C sulphates (contaminants in the clay) can decompose to produce gases; these reactions can also continue at higher temperatures before coming to a halt towards 1100 °C Should there be any calcium in the body new neo-formation phases such as Wollastonite, Ciehelenite, Diopside, Anorthite and quart/, may be found owing to the av ailability of silicon oxide in the system.
At 1050-1100 °C comes a key change: formation of Mull ite and Cristobalite (quartz crystallisation phases), which originate out of rearrangement of the crystalline structure of the Spinel. With its needle-like structure, Mullite gives the sanitaryware firmness at a moment in which its behaviour becomes pyroclastic (maximum firing temperature).
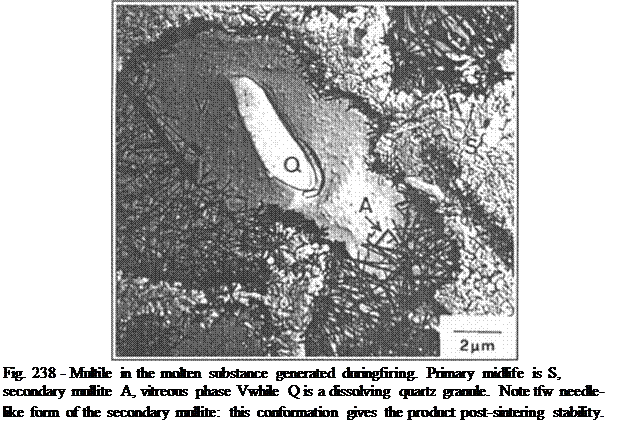
1120 °C is the temperature at which Albite melts, while at 1200 °C (pure) potassium feldspar melts: lx>th are present as fluxes. Their peculiarity is that they create eutectics with other mineral phases (i.e. mixtures with melting points that are lower than those of their individual components). Eutectics melt to create liquid inside the structure. This liquid acts as a catalyser for the reactions described above, aiding the migration of components and it is this molten substance that gives the body a pyro-plastic behaviour.
_
As temperature increases the viscosity of the molten material diminishes, so its mobility increases as do reaction rates. With melting comes the start of sintering: a rearrangement of the component particles that eliminates porosity, increases density and reduces its volume. Sintering frees air, which must then pass through the porosity of the glaze; melting of the latter must occur above the complete sintering temperature to prevent pin-holing. Sintering is a 3-stage process:
Initial stage: (up to .1% shrinkage) in which the spatial arrangement of particles becomes a set of uniform spheres linked together by necks';
Intermediate stage: (up to 92% of theoretical density) the system appears as a set of uniform grains joined at their faces and with cylindrical pores along the edges of the grains. The porous part is considered continuous at the beginning of the stage while at the end of it the pores tend to close;
Final stage: pores isolated inside the material mass.

Sintering is completed at temperatures of around 1200 °C and the holding time at maximum temperature w ill depend on the characteristics of the raw; materials and the particle size of the various body components. During the high temperature plateau the body completes the above-described transformations and it is at that temperature that the glaze needs to melt. As mentioned above, it is important that, during heating, the glaze maintains its porosity so that the gases produced by these reactions can filter through. It is also important that, during the maximum temperature plateau, the glaze is able to inter-penetrate the body so that stability of both components is total.
During the high temperature plateau any incorporated air bubbles are evacuated through the molten gla/.e to make the surface smooth.
Cooling is of fundamental importance in that the article has alreadv reached the mineralogical composition it will maintain throughout its lifespan; mineralisation and vitrification of the residual molten material also occurs, with consequent variations in mechanical properties. Cooling must take place fast enough to prevent de-vitrification of the glaze yet must not Ih* so fast as to aid the changes in volume caused by mineralogical transformations. Bear in mind that the transformation from Quartz a to Quartz (3 is particularly tricky as it involves the greatest variation in volume. The extent of contraction will be proportional to the quantity of residual quartz in the body and its particle size, which, if excessive, promotes contraction.
The tensions that build up at this time can cause damage even some time after the article has been fired.
| o | C . 0 - |
| at - | |
| r | |
| b | 22 - |
| i • a | 83 - |
| 2-: | |
| - | 35 - |
| - |
?C3
THERMAL EXPANSION CURVE
•JSC
dL/lo Sh.
6BC
'lo Sh.
IDENTITY No. 3236
DATE 5 0«: 2C"J2
OPERATOR Mores I
S«m.K 1 3653 STO v e -ti 26 INSTRUMENT DIL 482 EP / OIL. 2 ini SttftJE H. 012 03
■ft. -c9
I. Sh.
23 oc/;e.ac/:£Sje <jc
Fig. 2-fO - Thermit expansion curve. Noie the increase tn volume as temperature increase with the classic 'notch ' caused by reversible Quartz a - Quartz (3 transformation, Jollou-ed by; at about 10O0 °C, the decarbonation notch and, at 1100 °C, the start of sintering and the subsequent volume loss. When temperaturefalls to 573 CC the curve passes back through the Qiiartz P -Quartz a transfonnation. The body used for the folloni ng analyses is a standard I'itreous China.
| M m H | AN | ||
| m m m | MM | ||
| wk ft—if mpttm | |||
| UKMIOrr WW i —mi. r i l-in | tnoki carwuuKi |
2-*/ - Thermogravimetric Analysis (TG): noteflexion of the curve at th*point of organic substance oxidation. The dotted line is a derivative of the continuous one, the lower peak indicating maximum weight loss.

2+2 - Differential thermal analysis (DTA) highlights absorption and release of energy. Note the organic sutrslance oxidation peak at 342.2 °C. the absorption peak associated xcith the release of crystallised waterfrom the clays at 576.6 °C (this peak covers the snuiller, more tapered Quartz a- Quartz p transformation); the exothermic peak at .997.+ °C is caused by theformat ion of new mineral phases.
Date: 2015-04-20; view: 3627
| <== previous page | | | next page ==> |
| I 2.4 - GLAZING INNER SURFACES | | | FIRING CURVE |