
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Molarity (molar concentration)
Molarity (M) is the number of moles of solute in one litre of solution. To find the molarity of a solution one must know the molar mass of the solute. For example, “one-molar” solution of Sodium hydroxide (NaOH) contains one mole of NaOH in each litre of solution. The concentration of the proceeding solution is written as 1 M NaOH.
One mole of Sodium hydroxide (NaOH) has a mass of 40 g/mol (see Chapter 1.2). This quantity of NaOH dissolved in enough water to make exactly 1,00 L of solution gives a 1 M solution. If 20,0 g of NaOH, which is 0,500 mol, is dissolved in 1,00 L of solution, 0,500 M NaOH solution is produced:
Molarity = 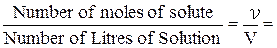
 =
=
= 0,500 M NaOH.
Note that one molar solution is not made by adding 1 mol of solute to 1 L of solvent. In such case, the final total volume would be slightly different from 1 L. Instead of this 1 mol of salute is firstly dissolved in less than 1 L of solvent. Then, the resulting solution is carefully diluted with more solvent to bring the total volume to 1 L, using volumetric flask.
Molarity is useful when the quantity of solute participating in a chemical reaction taking place in solution is of interest. Any required molar quantity of the solute can be selected by measuring out the appropriate volume of the solution of known molarity.
M = 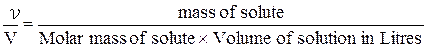 or
or
M = 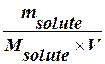 (7)
(7)
Normality (normal concentration)
The concentration of solutions can be expressed by stating the quantity of the solute in equivalents rather than in moles. This measurement of concentration is called Normality (N). The normality of solution is the number of equivalents (υE) of solute per litre (V) of solution:
Normality = 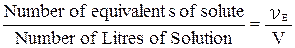 .
.
The normality of an acid or base solution is commonly expressed using the number of H+ or OH- ions available for a complete neutralization. Therefore:
Eacid = 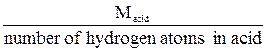 ,
,
Ebase = 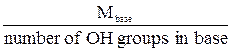 .
.
For neutral salts equivalent mass may be expressed as:
Eneutral salt = 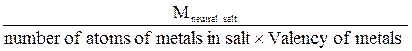 , where Eacid, Ebase, Eneutral salt - equivalent mass of acid, base or neutral salt respectively, g/g-eq;
, where Eacid, Ebase, Eneutral salt - equivalent mass of acid, base or neutral salt respectively, g/g-eq;
Macid, Mbase, Mneutral salt - molar mass of acid, base or neutral salt respectively, g/mol.
So, υE = 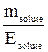 and
and
N =  (8)
(8)
Titre
Titre (T) is a special unit for measuring of concentration connected with chemical quantitative analysis. Titre is the number of grams of solute in 1 millilitre of solution. For example, the titre of a solution made from 0,3 g of Silver Nitrate (AgNO3) dissolved in 150 mL of solution is found as follows:
T = 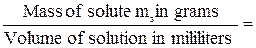
=  .
.
So,
T= 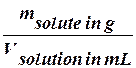 (9)
(9)
Table 11. Interconnection of concentration units
| Measuring unit | Formulas for re-calculation | ||||
| Name | Symbol | M | N | T | P |
| Molarity (molar concentration) | M | - | 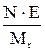
| 
| 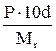
|
| Normality (normal concentration) | N | 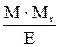
| - | 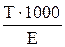
| 
|
| Titre | T | 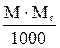
| 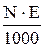
| - | 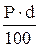
|
| Percent by Mass (mass concentration) | P | 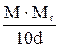
| 
| 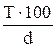
| - |
Mr - relative molar mass of solute, g/mol; 
E - equivalent mass of solute, g/g-eq; 
d - dencity of solution, g/L.
Date: 2015-01-12; view: 1206
| <== previous page | | | next page ==> |
| Concentration of solutions | | | Theory of dissociation |