
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Germanium, tin, and leadCompounds +2. There are oxides (GeO, SnO - black, PbO - yellow-red (litharge), hydroxides M(OH)2 - white. Salts of oxygencontaining acids are not typical for Sn2+ (and, especially, Ge2+). However, SnSO4 is used in electrolytic tinning[1]. EO oxides are produced by hydroxides decomposition when heated in a stream of N2: Å(OH)2 = ÅO + H2O (Å - Ge, Sn) PbO: 2Pb(NO3)2 = 2PbO + 4NO2 + O2 2Pb + O2 = 2PbO EO are insoluble in water, so E(OH)2 are obtained by the double replacement reactions of soluble salts with alkalis. EO and E(OH)2 are amphoteric since dissolve in acids and alkalis: Sn(OH)2 + 2HCl = SnCl2 + 2H2O Sn(OH)2 + 2NaOH = Na2[Sn(OH)4] In the series Ge—Sn—Pb basic properties of oxygen-containing compounds are enhanced, and acid properties are reduced: in case of Ge(OH)2 predominate acidic, and in Pb(OH)2 basic properties. For instance, Pb(OH)2 has K1basic = 10-5, and K1acid = 10-11.
Hydrolysis. Soluble E2+ salts hydrolyse in water. In connection with the strengthening of basic properties of hydroxides in the series Ge(OH)2 – Sn(OH)2 – Pb(OH)2 degree of hydrolysis of salts decreases. The lowest hydrolysis degree is observed in case of soluble salts of Pb: for example, Pb(NO3)3 gives acid reaction. Ge2+ salts almost completely decomposed by water in dilute solutions with the formation of Ge(OH)2. Salts of Sn (II) occupy an intermediate position: SnCl2 + H2O Û SnOHCl¯ + HCl HCl solution is added to avoid formation of basic salt precipitate. Insoluble in water compounds are: PbSO4, PbCrO4, PbBr2, PbI2, with low solubility - PbCl2. The soluble salts are nitrate, acetate, hexafluorosilicate. Pb(CH3COO)2 has a sweet taste and is called lead sugar. All salts of lead are poisonous. Red-Ox. Oxidation state (+2) is an intermediate state between typical for these elements 0 and (+4), so they can both oxidants and reductants. Oxidising properties are expressed very poorly, (Åî(Ge2+/Ge) = 0,2 V, Åî(Sn2+/Sn) = -0,136 V, Åî(Pb2+/Pb) = -0,126 V, which are close to 0). Examples of these E2+ properties are given below: PbO + CO = Pb + CO2, or SnCl2 + Zn = Sn + ZnCl2 Pb(CH3COO)2 + Fe = Pb + Fe(CH3COO)2 Reducing properties in the series Ge—Sn—Pb diminish. They are expressed more strongly in alkaline medium than in acidic (compare the standard potentials): Å°Sn4+/Sn2+ = + 0,15 V; E° Sn(OH)6-2/Sn(OH)4-2 = -0,9 V Å°Pb4+/Pb2+ (H+)= + 1,68 V; E° PbO2/Pb(OH)2(OH-)= +0,28 V SnCl2 is often used in practice of chemists as a chemical reductant: SnCl2 + 2FeCl3 SnCl2 + HgCl2 3SnCl2 + 2BiCl3 + 12NaOH = 2Bi¯ + 3Sn(OH)4¯ + 12NaCl 3GeCl2 + 2K2Cr2O7 + 16HCl = 3GeCl4 + 2CrCl3 + 4KCl + 8H2O Pb2+ is a very weak reductant. Therefore, it can be oxidised only by the strongest oxidants in alkaline medium: Pb(OH)2 + Cl2 + 2NaOH = PbO2 + 2NaCl + 2H2O Na2[Pb(OH)4] + Br2 + 2NaOH = PbO2 + 2NaBr + 2H2O
Compounds (+4). Production. GeO2 and SnO2 are produced by the direct combination of elements. PbO2 is obtained in the laboratory by Pb(CH3COO)2 oxidation with chlorinated lime: Pb(CH3COO)2 + CaOCl2 + H2O = PbO2¯ + CaCl2 + 2CH3COOH
Properties. GeO2 is an acid oxide. Unlike SiO2, SnO2, and PbO2, it is slightly soluble in water (4 g/l), forming hydrates of varying composition, called germanic acid. Meta-H2GeO3 and ortho-H2[Ge(OH)6] are known. This is a weak acid: H2GeO3 Û H+ + HGeO3- K1 = 1.10-9 HGeO3- Û H+ + GeO32- K2 = 2.10-13 However, GeO2 displays amphoteric properties (reacts with alkalis, forming hydroxogermanates) GeO2 + NaOH + 2H2O = Na2[Ge(OH)6], and when heated with concentrated HCl it forms GeCl4: GeO2 + 4HCl = GeCl4 + H2O SnO2 is a polymeric inactive substance. It does not react with acids and alkali solutions, but reveals amphoteric properties (when heating, Í2SO4(conc.) or fusion with alkalis): SnO2 + 2Í2SO4(conc.) = Sn(SO4)2 + 2H2O SnO2 + 2NaOH = Na2SnO3 + H2O Tin (+4) hydroxide can be prepared by the double replacement reaction of soluble compounds: SnCl4 + 4NaOH = Sn(OH)4¯ + 4H2O This white amorphous precipitate is a polymer of varying composition (SnO2)õ∙(Í2Î)y. Some simplified formulae can be offered: H2SnO3 or SnO(OH)2 (x = 1, y = 1); H4SnO4 îr Sn(OH)4 - ortho-form (x = 1, y = 2). Freshly prepared precipitate is called a-stannic acid. It has amphoteric nature and reacts with alkalis and acids easily: Sn(OH)4 4HCl = SnCl4 + 4H2O Sn(OH)4 + 2NaOH = Na2[Sn(OH)6] When standing (sooner when heating), the process of Sn(OH)4 “aging” takes place, transforming into inactive form - b-stannic acid. The process of aging illustrates the following scheme: PbO2. There are two polymorphs: orthorhombic a-PbO2 and tetragonal b-PbO2. b-PbO2 is the most stable at normal conditions. b-PbO2 ® a-PbO2 transition occurs at 300 oC and pressure > 13 000 atm. At atmospheric pressure b-PbO2 decomposes at 280 oC (a-PbO2 at 220 oC): 3PbO2 = Pb3O4 + O2 Preparation. 1. Action of HNO3 on Pb3O4: Pb3O4 + 4HNO3 = Pb(NO3)2 + PbO2 + 2H2O not H4PbO4 2. Anode oxidation of Pb2+ salts: PbSO4 + 2H2O -2e ® PbO2 + H2SO4 + 2H+ 3. the action of strong oxidants on them: Pb(CH3COO)2 + Cl2 + 2H2O = PbO2 + 2CH3COOH + 2HCl Properties. PbO2 is insoluble and does not react with water. This is an amphoteric oxide and, like SnO2, it reacts with acids and alkalis at rigid conditions. The difference is that the salts formed, for example, Na2PbO3 or Pb(SO4)2 do not exist in aqueous solutions and hydrolyse completely. PbO2 is not soluble in dilute HÑl, H2SO4, HNO3, but it dissolves in a mixture of diluted HNO3 + H2O2, and concentrated HÑl, Í2SO4: PbO2 + 2HNO3 + H2O2 = Pb(NO3)2 + O2 + 2H2O PbO2 + 4HCl(conc.) = PbCl2 + Cl2 + 2H2O (like MnO2) 2PbO2 + 2H2SO4(conc.) = PbSO4 + O2 + 2H2O Fine precipitate of PbO2 is soluble in alkali solutions: PbO2 + 2NaOH + 2H2O ® Na2[Pb(OH)6]
PbO2 is a very strong oxidant (in acidic medium): 5PbO2 + 2MnSO4 + 3H2SO4 = 5PbSO4 + 2HMnO4 + 2H2O PbO2 + Cr3+ + H2O Hydrated PbO2 form does not exist (they are extremely unstable), i.e. Pb(OH)4 or plumbic acids are not known. However, salts of these acids (plumbates) can be obtained for many metals, including Pb2+: PbO + PbO2 = PbPbO3 (Pb2O3) Pb(II) meta-plumbate 2PbO + PbO2 = Pb2PbO4 (Pb3O4) Pb(II) ortho-plumbate Pb3O4 is called red lead and used as a red-orange pigment. It can be prepared by heating PbO in air:
Pb3O4 reacts with strong acids; its oxidation state does not change, confirming that Pb3O4 can be attributed to salts: Pb2PbO4 + 4HNO3 = 2Pb(NO3)2 + H4PbO4 ® PbO2 + 2H2O (immediate decomposition) Pb3O4 dissolves in alkalis: Pb2PbO4 + 6NaOH + 4H2O = 2Na2[Pb(OH)4] + Na2[Pb(OH)6] This is a strong oxidant: Pb3O4 + 8HCl = 3PbCl2 + Cl2 + 4H2O In summary, Ge subgroup oxides stability can be described by the scheme:
oxidizing properties growth
stability growth oxidation reduction
GeO SnO PbO reducing properties growth
Thus, there is an increase of the lowest oxidation state stability in accordance with amplification of metallic propertiesin the series Ge—Sn—Pb.
Date: 2016-01-03; view: 1057
|
 2FeCl2 + SnCl4
2FeCl2 + SnCl4
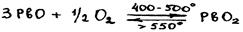
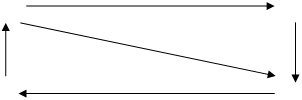 GeO2 SnO2 PbO2
GeO2 SnO2 PbO2