
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
OXYGEN CONTAINING COMPOUNDSSilicon Silicon dioxide. Si interacts with O vigorously when heated: Si + O2 = SiO2 DH°298 = -908 kJ / mol SiO2 structure.Natural SiO2 occurs in crystalline (quartz) and amorphous forms. Crystalline SiO2 exists as a set of several polymorphs, which are mutually transformed from one into another in accordance with the following scheme:
575 º 130 º 270 º <1650 º
α-quartz α-tridymite α-cristobalite glass
Quartz, tridymite, and crystobalite can have mutual transitions from one to another, but they are very slow. Consequently, tridymite, and crystobalite can be stored very long time at room temperature and exist as separate minerals despite their thermodynamic instability. Note that a-modifications are stable at room temperature and b-modifications at high temperature. At the rapid cooling of the melt, crystallization has not time to occur and amorphous vitreous form, fused silica (quartz glass) appears. Various modifications of SiO2 are inorganic polymers with heterochains.
These giant molecular entities have strong covalent bonds SiOSi. Each Si atom is surrounded by tetrahedron of four O atoms and O atoms, in turn, connect to each other two such tetrahedrons. These connected tetrahedrons twisted in a spiral like staircase of the type of screw. Spirals have parallel orientation in space. The crystalline form with rightscrew spirals is called a-quartz, and with leftscrew spirals is called b-quartz. Properties. Polymeric SiO2 is not soluble in water at STP. It is stable to attacks of acids. It dissolves only in hydrofluoric acid, HF, due to the formation of volatile SiF4 with stronger SiF than SiO bonds: SiO2 + 4HF = SiF4 + 2H2O SiO2 dissolves slowly in alkali solutions, and quickly in their melts forming silicates: SiO2 + 2NaOH = Na2SiO3 + H2O SiO2 is a silicic acid anhydride. As a compound with extremely low volatility (fugitivity), it displaces other acid anhydrides when melting with salts that are used in glassmaking: SiO2 + Na2SO4
Silicic acids. The overwhelming majority of meta-silicic acid, H2SiO3, salts are insoluble in water. Na2SiO3 is one of the few soluble salts. It is called soluble glass and aqueous solutions of the salt liquid glass. H2SiO3 is a very weak acid. Its soluble in water fraction of molecules dissociates in negligible small degree (K1 = 1 ∙ 10-10). Liquid glass forms strong alkaline medium and high viscosity after hydrolysis due to the formation of polymorphs of orthosilicic acid (silicate glue): Na2SiO3 + H2O = Na2H2SiO4 Na2H2SiO4 + 2H2O = H4SiO4 + 2NaOH Some salt H2SiO3 with weak bases completely hydrolyze in a solution. Therefore, they cannot be obtained by the double replacement reaction: Na2SiO3 + 2NH4Cl + H2O = H4SiO4 + NaCl + 2NH3 Metasilicic acid is displaced from its salts in aqueous solutions by other acids, including H2CO3. At first, water-soluble orthosilicic acid appears which can exist in very dilute solutions: Na2H2SiO4 + 2HCl = H4SiO4 + 2NaCl
The growth of concentration or just standing leads to polymerization:
The elementary block of such a chain polymer is H2SiO3, but the further condensation leads to branched, cross-linked, bulk polymers, and, eventually, polymeric SiO2 due to transformations of OH-groups:
Si + Si + -H2O
Si OH OH H - O O O
H O Si O Si OH -H2O Si Si
OH OH H O O O Si HO OH
Tetrahedral surrounding of silicon by oxygen atoms occurs in any polymeric form of silicic acid. These polymers are insoluble and have nonstoichiometric composition (SiO2)x∙(H2O)y. When x> 1 they correspond to polysilicic acids, which derivatives is a variety of natural silicates. Silicate materials are produced artificially in large quantities. The maximal amount among synthetic silicates has glass, which composition expresses the formula Na2CaSi6O14 or Na2O∙CaO∙6SiO2. The partial replacement of Na, Ca, Si atoms by the atoms of other elements gives a great number of different kinds of special glass depending on the nature of its application. For example, laboratory glassware contains significant content of B2O3 and Al2O3. It is characterized by high chemical stability (especially, in acidic medium) and low coefficient of thermal incandescences.
Silicon halides. Silicon forms halides of general formula SiX4 with halogens: SiO2 + 2C + 2Cl2 Tetrahalides are very reactive substances. They hydrolyze easily as halogenanhydrides of ortho-silicic acid: SiCl4(aq) + 4H2O = H4SiO4(aq) + 4HCl(aq) DG°298 = -238 kJ / mol Hydrolysis is a result of consecutive H2O molecules addition and HX molecules removal up to H4SiO4 formation. SiX4 fumes in air due to hydrolysis (e.g. SiCl4 is used to create artificial fog). SiCl4 is an active chlorinated agent that interacts with oxides of metals and nonmetals: 3 SiCl4 + 2Al2O3 = 3SiO2 + 4AlCl3 SiF4 interacts with HF acid forming hexafluorosilicic acid, H2SiF6: SiF4 + 2HF = H2[SiF6] Si atom in H2SiF6 is in sp3d2-hybrid state and its coordination number is 6. H2SiF6 is not known in the free state, it is a strong acid (» H2SO4). Other silicon tetrahalides do not form hexahalido compounds. Their resistance to various reagents and heating decreases toward SiI4. Silicon nitride. Amorphous Si interacts with N2 only at t>1300 °C. Silicon nitride, Si3N4, formed is a solid stable compound that only decomposes slowly by molten alkali or hot concentrated HF: Si3N4 + 12NaOH = 3Na2SiO3 + 4NH3 Si3N4 + 16HF = 2(NH4)2SiF6 + SiF4 Silicon carbide. Si or SiO2 react with carbon in electric furnaces at t » 2300 °C. SiC is a very hard (the second after diamond) refractory material. It is chemically stable at normal conditions, but decomposes when heated in molten alkali (in the presence of O2), Cl2, hot steam: SiC + 4NaOH + 2O2 = Na2SiO3 + Na2CO3 + 2H2O ¯8e 2e×2 SiC + 2Cl2 SiC + 2H2O CHEMICAL COMPOUNDS Date: 2016-01-03; view: 989
|
 β-quartz 870 º β-tridymite 1470 º β-cristobalite 1723º liquid
β-quartz 870 º β-tridymite 1470 º β-cristobalite 1723º liquid
 Na2SiO3 + SO3
Na2SiO3 + SO3

 H O O H H - O O H
H O O H H - O O H H O O H H O O H . . .
H O O H H O O H . . . HO OH
HO OH
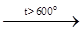 SiCl4 + C
SiCl4 + C SiO2 + CH4
SiO2 + CH4