
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Silicon and germanium
Silicon is a hard, dark-gray nonmetallic element. It is very abundant, making up about 28 per cent of the earth's crust. Only oxygen is more plentiful. Silicon occurs exclusively in compounds, combined chiefly with oxygen in many types of silicates. There are more than 800 known natural silicates.
Silica, a type of silicate, is the main ingredient of sand. It is also a major ingredient of quartz, lava, and various minerals that form rocks such as granite and sandstone. A new form of silica was produced when the first atomic bomb was exploded in New Mexico.
Pure silicon is indispensable to the electronics industry because it is a good semiconductor. A semiconductor conducts electricity with an efficiency between that of conductors (like metal) and insulators (like glass). This allows for greater control in transmitting electric signals. Thus, pure silicon is used in making integrated circuits, solar cells, transistors, and similar devices that transmit electric sig-
nals. In 1950, 2 ounces (56 grams) of extremely pure silicon produced 50 transistors. In 1980, the same amount provided one million transistors, as integrated circuits. A single integrated circuit can now hold more than 10,000 transistors and still efficiently and accurately control electric signals.
Silicon compounds are also widely used in industry—in making glass, in grinding and polishing other materials, in producing synthetic rubber, and in making lubricants, insulators, and water repellents.
Germanium is a hard, brittle, grayish-whitfe metal. It is comparatively rare, being found in small amounts in copper, lead, and zinc ores. Germanium, like silicon, is widely used as a semiconductor, especially in diodes and solar batteries. It is also used in certain kinds of telephone lines, data transmission lines, and computer cables, as well as in some lead-acid storage batteries, and in medicine.
Tin
Tin is a malleable white metal that is found primarily in the southern hemisphere. It makes up only about .001 per cent of the earth's crust. Malaysia is the world's leading producer of tin.
Tin was one of the earliest metals known and its use has closely followed the growth of civilization. Food canning and preservation developed with tin-coated steel. Fast machinery depended on tin-based bearings. Printing expanded with metal type that contained tin. Many communications devices first depended on tin-based solder (an alloy used to join metal surfaces). Tin alloys are used for musical instruments and, especially, organ pipes. Because the metal is comparatively rare in nature, it is now sufficiently expensive for tin cans to be collected and the tin extracted and recycled.
Tin can be easily formed into complex shapes. It also protects steel from rusting. For these and other reasons, tin is chiefly used today to make tin plate. Tin plate is a very thin film of tin used to coat both sides of a piece of steel. Tin-plated steel is used chiefly for making tin cans. Tin coatings are also applied to staples, pins, paper clips, and containers and utensils for handling and storing food.
Tin is a major ingredient of solder (tin and lead), bronze (tin and copper), and pewter (tin, antimony, and copper). Small amounts of tin also go into the making of bearings, dental fillings, toothpastes, printing alloys, and cast iron.
Lead
Lead is a soft but heavy bluish-gray metal. It is widely distributed in the earth's crust, usually found in a gray metallic ore called galena. Galena most often contains other metals also, such as copper, gold, silver, and zinc. The world's leading lead-mining countries are Australia and the Soviet Union.
Lead is very soft and malleable. It is easily cast, joined, and converted into pipes or sheets. The principal modern use of the metal is in the electrodes of storage batteries. These batteries provide power for the electrical systems of automobiles, airplanes, and many other vehicles. Lend is also added to gasoline
Major groups of elements: Silicon to lead 39

|
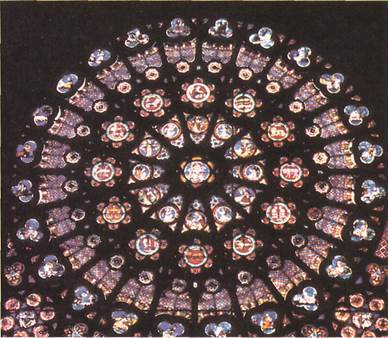
The eroded sandstone pinnaclesof Bryce Canyon in the United States (left) and the stained glass window(below) both consist largely of silica. In the sandstone, the silica is in the form of quartz. In the stained glass, it is mixed with lead oxide. Most old stained glass also contains various metal compounds to make it colored. Gold, for instance, produces red glass.

|
|
| to improve the performance of certain automobile engines. Lead alloys and compounds have many uses. They are used in making printing type, bearings, solders, insecticides, explosives, and rubber products. Because lead is the densest common metal, it is used as ballast weight (for stabilizing and balancing objects) and as shielding against X rays and gamma rays. Lead's resistance to corrosion makes it invaluable in certain paints and dyes applied to bridges and other steel structures. It is used to make cable coverings that protect telephone and power lines. It is also used to make pipes, storage tanks, and other equipment for shipping and storing certain corrosive chemicals. Lead is very poisonous. Particles of lead can enter the body through the lungs or the skin, or by swallowing. Lead may damage internal organs like the kidneys, the brain, or the liver. It also disrupts the production of red blood cells. For these reasons, the amount of lead in paint and gasoline is now restricted. The amount of lead that can be released into the air by industrial plants is also strictly regulated. |
| to improve the performance of certain automobile engines. Lead alloys and compounds have many uses. They are used in making printing type, bearings, solders, insecticides, explosives, and rubber products. Because lead is the densest common metal, it is used as ballast weight (for stabilizing and balancing objects) and as shielding against X rays and gamma rays. Lead's resistance to corrosion makes it invaluable in certain paints and dyes applied to bridges and other steel structures. It is used to make cable coverings that protect telephone and power lines. It is also used to make pipes, storage tanks, and other equipment for shipping and storing certain corrosive chemicals. Lead is very poisonous. Particles of lead can enter the body through the lungs or the skin, or by swallowing. Lead may damage internal organs like the kidneys, the brain, or the liver. It also disrupts the production of red blood cells. For these reasons, the amount of lead in paint and gasoline is now restricted. The amount of lead that can be released into the air by industrial plants is also strictly regulated. |
| Galena,the silver-gray crystals in the photograph (left), is the principal ore of lead. It is one of the most widely distributed sulfide ores. It occurs in many different types of rocks. In addition to lead, some galena deposits contain silver. Galena is therefore also mined as a source of silver. |
 Fact entries
Fact entries
Siliconwas first isolated in 1823 by the Swedish chemist Jons Berzelius (1779-1848). Its name is derived from the Latin si/ex, meaning flint. At. no. 14; at. mass 28.0855; m.p. 1410° C; b.p. 2355' C.
Germaniumwas predicted in 1871 by the Russian chemist Dmitri Mendeleev (1834-1907). It was not discovered until 1886 by the German chemist Clemens Winkler (1838-1894). He named it after his homeland.
At. no. 32; at. mass 72.59; m.p. 937.4° C; b.p. about 2830° C.
Tin,in the form of bronze (an alloy with copper), has been known since at least 3500 B.C. Its chemical sym-
bol, Sn, comes from the Latin word for the element, stannum. One naturally occurring form of tin—gray tin—is powdery at low temperatures. At. no. 50; at. mass 118.71; m.p. 231.9° C; b.p. 2270° C.
Leadhas been known as a metal since at least 4000 B.C. Its chemical symbol, Pb, is derived from the Latin word for the element, plumbum. At. no. 82; at. mass 207.19; m.p. 327.5° C; b.p. 1740° C.
 |

|
| 3B | 4B | 5B | 6B | 7B | r | --- *- | > | 1B |
| Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu |
| 44.9559 | 47.88 | 50.9415 | 51.996 | 54.938 | 55.847 | 58.9332 | 58.69 | 63.546 |
| '••6 | ||||||||
| Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag |
| 88 9059 | 91.224 | 92,9064 | 95.94 | (98) | 101.07 | 102.906 | 106.42 | 107.868 |
| La | Hf | Ta | w | Re | Os | lr | Pt | Au |
| 138.906 | 178.49 | 180,948 | 183.85 | 186.207 | 190.2 | 192.22 | 195,08 | 196.967 |
| Ac | ||||||||
| 227.028 |
| The transition metals comprise 21 elements. These occupy the central block of the periodic table, from Group 4B to Group 8 (excluding elements 104 through 109). They have several general features in common, including the formation of compounds in different oxidation states and the tendency to show catalytic activity. The metals of the first transition series— from titanium to nickel—are most notable for their industrial importance. |
Date: 2015-12-11; view: 1802
| <== previous page | | | next page ==> |
| Gallium, indium, and thallium | | | The first transition series |