CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Copper, silver, and goldSometimes called the coinage metals, copper (Cu), silver (Ag), and gold (Au) make up Group 1B of the periodic table. These metals are the best conductors of heat and electricity. They are also the most malleable and ductile of all metals. They can be hammered into extremely thin sheets without breaking or drawn into wire thinner than human hair without cracking. They are very resistant to corrosion. Each metal also has a range of other valuable features, both individually and as alloys and compounds. Copper Copper is a reddish-orange metal that has been known for about 10,000 years. It is found on every continent, but especially in North and South America and in central Africa. In its raw state, copper ore contains about 4 per cent pure copper. It is the principal nonferrous metal. This is a metal that contains no iron. It is also one of the few common metals used mainly in the pure form, rather than as alloys (combinations of metals)—especially in electrical appliances. During the production process, raw copper ore is crushed, mixed with water, refined by the temporary addition of chemicals, smelted, and finally purified by electrolysis (often also yielding nickel, silver, and other precious metals). Electrolysis is a process that sends an electric current through the molten copper, producing chemical reactions, which yield copper metal that is over 99.9 per cent pure. As a conductor of electricity, only silver is better than copper. However, because silver is too expensive, copper is far more extensively used. About six-tenths of copper production is used by the electrical industry, chiefly in the form of wire. Copper wire is used in electrical wiring, telephone and sound systems, televisions, motors, and various other kinds of electrical equipment. Although not as malleable as silver or gold, copper can still be worked into sheets less than one five-hundredth of an inch (.05 millimeter) thick. Copper wire can be drawn thinner than human hair. The characteristic green film that appears on copper after long exposure is called a patina. It preserves the metal against further corrosion. Copper never rusts.
Important copper alloys include cupro-nickel for coins; brass (copper and zinc) for electrical fixtures, hardware, and metal decoration; and bronze (copper and tin) for bells, sculpture, ornaments, tools, and vases. Silver Silver is a soft, white metal that has been used for jewelry, money, and religious purposes for about 6,000 years. Silver and silver ore are found in most countries. The leading silver-mining countries are Mexico, Peru, the Soviet Union, and the United States. Most silver has to be extracted from ores. Pure silver that occurs naturally accounts for only about 20 per cent of all silver produced annually. Because pure silver is extremely soft, it is usually combined with another metal for strength and hardness. For example, 7.5 per cent copper is added to 92.5 per cent silver to make the alloy sterling silver. Another alloy-coin silver (90 per cent silver, 10 per cent copper)—is no longer used for coins, but employed for electrical contacts. Silver is the best conductor of heat and electricity. It is used in the electrical and electronic equipment industries whenever the need for superior conductivity outweighs silver's high cost. The ability of silver to be hammered into different shapes or drawn into very fine wires is second only to gold. Many art objects are crafted from silver, including religious arti-
Two micrographsshow silver halide crystals magnified about 3,750 times. To the near right is conventional photographic film and to the far rights new ultrasensitive film. The new film is more sensitive because the flat, regular shape of its large silver halide crystals enables them to absorb more light than the irregular, boulderlike crystals of the other film. Major groups of elements: Copper, silver, and gold 29
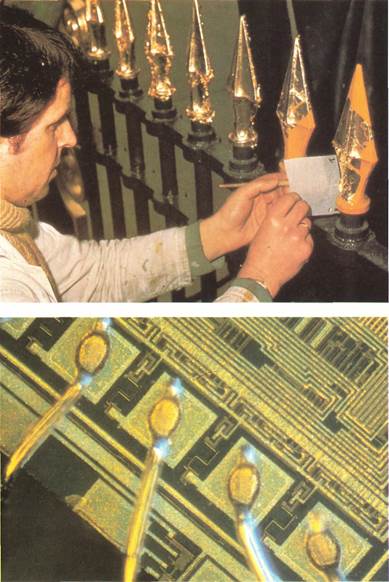
Cold,the most malleable of all metals, can be beaten into extremely thin leaves. Gold leaf is used to embellish a variety of objects, from iron railings (right)'to the covers of special-edition books. facts, tableware, and jewelry. Although silver does not rust, it does react with sulfur compounds in the air and develop a black or gray coating of silver sulfide. This is called tarnish and is a bigger problem today than ever, because of the presence of sulfur compounds in polluted air. Silver is also use'd by doctors and dentists. Certain surgical instruments are made of it, because silver helps to kill bacteria. Cavities are filled with a mixture of silver, tin, and mercury. there are many compounds of silver. Among them are silver nitrate, which is soluble in water and used to make silver plate and silver mirrors. Silver oxide is used in small, powerful batteries for hearing aids, timepieces, and calculators. Silver bromide plays a major role in photography, being the light-sensitive element in photographic film. Gold With its soft, metallic glow and untarnishable yellow color, gold has been mined, used, and treasured by mankind since prehistoric times. Cold coins, jewelry, and emblems form an important part of recorded history. Gold is accepted by all countries as a form of payment for international debts. Gold is widely distributed in small amounts, usually combined with other metals (such as silver, zinc, or copper) into alloys. In the United States, only about 40 per cent of the gold produced annually is mined from the pure state. Gold is the most malleable and ductile of all metals. Gold leaf, which transmits green light but appears golden in reflected light, is made by carefully beating the metal. It can be as thin as one two-hundred-thousandth of an inch (.000127 millimeter). It is used for gilding and in the arts for lettering. Because gold is extremely soft, it is usually alloyed with copper, silver, zinc, or nickel when used in making jewelry. The proportion of gold in alloys is often expressed in carats (sometimes spelled karats). Pure gold is 24 carats (24K). Therefore, 18-carat gold is three-fourths gold and one-fourth other metals. A compound called white gold is made from gold alloyed with another metal such as silver, platinum, or palladium. It is often used as ring settings for precious stones. Although expensive, gold is increasingly used to plate contacts in electronic circuits. The electrical contactsof microchips (above) and other electronic devices are often plated with gold. Cold is not only a good electrical conductor, but also protects the contacts from corrosion. This improves reliability.
Fact entries Copperwas first used by late Stone Age man around 8000 B.C. Its name and its chemical symbol (Cu) derive from the Latin word for the metal, cuprum. It occurs naturally as the free metal in basaltic lavas and also as copper compounds in many minerals. At. no. 29; at. mass 63.546; m.p. 1083.4° C; b.p. 2567° C. Silverornaments dating from about 4000 B.C. have been found in royal tombs. Its chemical symbol, Ag, is derived from the Latin word for the metal, argentum. It occurs naturally in the free metallic state and as compounds in various minerals. At. no. 47; at. mass 107.868; m.p. 961° C; b.p. 2193° C. Goldhas been known since prehistoric times. Because it does not corrode and mainly occurs naturally in a relatively pure form, it was one of the first metals used by people. Its chemical symbol, Au, comes from the Latin word for the metal, aurum. At. no. 79; at. mass 196.967; m.p. 1064.43° C; b.p. 2807° C.
The alkaline earth elementsmake up Croup 2A of the periodic table. Chemically, they resemble, but are less reactive than, their counterparts in Croup 1A. The alkalines have little similarity to their other neighbors in Group 3B. Alkaline earths The six elements of Group 2A of the periodic table are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). They are known as the alkaline earth metals because their oxides (metals combined with oxygen) are alkaline, meaning they neutralize acids and form salts with them. All of the metals react with water and, except for radium, are of industrial importance. They form a closely related group of highly metallic elements with similar physical properties. These metals are never found in nature in their pure form. They have to be separated from the minerals that contain them. Date: 2015-12-11; view: 1389
|