
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Green Chemistry
Yet as cancer rates rise and evidence increases about the link between certain chemicals and birth defects and learning disabilities our regulatory system has been unable to make chemical producers provide full testing information or promote inherently safer chemicals. While some efforts are underway to overhaul chemicals policy, most notably by the recent passing of the European Union’s new chemicals policy, REACH, the focus must also be on overhauling the way chemicals are designed from the outset. This is what Green Chemistry sets out to do. What is Green Chemistry? Green chemistry is an approach to the design, manufacture and use of chemical products to intentionally reduce or eliminate chemical hazards. The goal of green chemistry is to create better, safer chemicals while choosing the safest, most efficient ways to synthesize them and to reduce wastes. How Is Green Chemistry Different? Chemicals are typically created with the expectation that any chemical hazards can somehow be controlled or managed by establishing “safe” concentrations and exposure limits. Green chemistry aims to eliminate hazards right at the design stage. The practice of eliminating hazards from the beginning of the chemical design process has benefits for our health and the environment, throughout the design, production, use/reuse and disposal processes. In 1998, two US chemists, Dr Paul Anastas and Dr John Warner outlined Twelve Principles of Green Chemistry to demonstrate how chemical production could respect human health and the environment while also being efficient and profitable. The 12 principles are: 1. It is better to prevent waste than to treat or clean up waste after it is formed. 2. Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product. 3. Wherever practicable, synthetic methodologies should be designed to use and generate substances that possess little or no toxicity to human health and the environment. 4. Chemical products should be designed to preserve efficacy of function while reducing toxicity. 5. The use of auxiliary substances (e.g. solvents, separation agents, etc.) should be made unnecessary wherever possible and innocuous when used. 6. Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperature and pressure. 7. A raw material or feedstock should be renewable rather than depleting wherever technically and economically practicable. 8. Reduce derivatives - Unnecessary derivatization (blocking group, protection/ deprotection, temporary modification) should be avoided whenever possible. 9. Catalytic reagents (as selective as possible) are superior to stoichiometric reagents. 10. Chemical products should be designed so that at the end of their function they do not persist in the environment and break down into innocuous degradation products. 11. Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances. 12. Substances and the form of a substance used in a chemical process should be chosen to minimize potential for chemical accidents, including releases, explosions, and fires. One example of the difference between traditional chemistry and green chemistry is the use of petroleum. Today’s chemical industry relies almost entirely on non-renewable petroleum as the primary building block to create chemicals. This type of chemical production typically is very energy intensive, inefficient, and toxic - resulting in significant energy use, and generation of hazardous waste. One of the principles of green chemistry is to prioritize the use of alternative and renewable materials including the use of agricultural waste or biomass and non-food-related bioproducts. In general, chemical reactions with these materials are significantly less hazardous than when conducted with petroleum products. Other principles focus on prevention of waste, less hazardous chemical syntheses, and designing safer chemicals including safer solvents. Others focus on the design of chemicals products to safely degrade in the environment and efficiency and simplicity in chemical processes. A transformation to green chemistry techniques would result in safer workplaces for industry workers and safer products for consumers. And because green chemistry processes are more efficient companies would consume less raw materials and energy as well as save money on waste disposal. How to Design Safer Chemicals The more we know about how a chemical’s structure causes a toxic effect, the more options are available to design a safer chemical. Chemists now have access to many sources of information to determine the potential toxicity of the molecules they design and the ingredients they choose. Green chemists are trained to integrate this information in to the design of molecules to avoid or reduce toxic properties. For example, they might design a molecule large enough that it is unable to penetrate deep in to the lungs, where toxic effects can occur. Or, they might change the properties of a molecule to prevent its absorption by the skin or ensure it safely breaks down in the environment. Adopting Green Chemistry
Comprehension Aspect
Ex.1. Say whether the following statements are true or false. Discuss them with your neighbour. Correct the false ones. 1. Most synthetic industrial chemicals remain untested for their health effects. 2. One of the principles of green chemistry is to prioritize the use of alternative and renewable materials. 3. Synthetic methodologies should be designed to use and generate substances that possess little or no toxicity to human health and the environment. 4. Green chemistry must be aimed to eliminate hazards at the stage of disposal. 5. The most fundamental principle of green chemistry is that the best way to prevent harmful chemical pollution is to design materials that are inherently environmentally benign and safe for human health. Ex.2. Make a plan for the discussion of the text.
Ex.3. Find some facts or ideas in the text to support the points of your plan. Ex.4. Split into groups or pairs and discuss the following questions. Make your own research, find out the necessary information. 1. Why has green chemistry gradually become recognized as both a culture and a methodology for achieving sustainability? 2. What are the main goals of green chemistry? 3. What 12 Principles were outlined in 1998? 4. How can chemists design safer chemicals? 5. What benefits do green chemistry technologies provide? The following words and word combinations will help you to answer the question. (reduced waste, safer products, healthier workplaces and communities, reduced use of energy and resources, improved competitiveness of chemical manufacturers and their customers, fewer accidents…).
GRAMMAR ASPECT
CONDITIONAL SENTENCES
Ex.1. Study the following chart carefully.
Notes 1. Unreal conditionals can be also expressed in the following way: a) But for + noun/pronoun But for the rain, we would go to the country.
b) If it were not for + noun/pronoun If it were not for the rain, we would go to the country. 2. Adverbial clauses of condition containing had, were, could, should are introduced without any conjunction to make the sentences more emphatic. In these cases we find inversion: If I had time, I would come over. Had I time, I would come over.
If I were at your place, I would call him. Were I at your place, I would call him.
Ex. 2. a) Tell what you will do if you are free on Sunday. (Type I). Use the suggestions given below. to go on a trip to Vilnius, to go to the cinema (theatre), to read up for the examination, to go on an excursion, to study the lessons carefully, ( your idea). b) Tell what you would do if you did not understand some phenomenon. (Type II). to ask one of the friends to explain it, to try to understand by oneself, to ask the teacher to explain it, to read the necessary literature about it.
c) Tell what you would have done if you had had much time last month. (Type III). to go hiking, to complete the experiment, to finish the research work, to go on an excursion to Moscow, (your suggestion).
Ex. 3. Change the following sentences of real condition a) to unreal condition I, b) to unreal condition II. 1. If anybody asks for you, I will tell him to call back later. 2. If you study these lessons carefully, you will be able to answer all the questions. 3. If he asks me, I will gladly help him. 4. The teacher will get angry with me if I make that mistake again. 5. If the experiment is interesting, I will carry it out.
Ex. 4. Use the correct tense form of the verb in brackets in each sentence. 1. He would have spoken to Mr. Carrington if he (to see) him yesterday. 2. If she (not to know) the answer, she always admits it right away. 3. I (to believe) it if I had seen it with my own eyes. 4. But for your advice, I (not to be able to) solve my problems. 5. If you (to go on) making so much noise, I will send you out. 6. Provided zinc were heated with sulphuric acid, the metal (to replace) hydrogen. 7. The study of any substance (to be) incomplete unless we investigated the properties of that substance and the methods of obtaining its components.
Ex. 5. Substitute the word ``unless`` for ``if…not``. Study the Model.
1. She wouldn’t do that if she were not allowed to. 2. If you don’t study harder, you are going to fail at the examination. 3. If you don’t understand my instructions, read them again. 4. If iron is not heated red-hot, it won’t absorb any carbon. 5. If he hadn’t followed the instructions, he wouldn’t have obtained good results.
Ex. 6. State the type of each conditional sentence. Translate these sentences into Russian. 1. If the gas were pure, we could immediately use the Gas Law to calculate the number of moles produced by the reaction. 2. If Gibbs had never published another paper, this single contribution ``On the Equilibrium of Heterogeneous Substances`` would have placed him among the greatest theoreticians in the history of science. 3. The collisions of the molecules result in no loss of energy. If energy were lost, the temperature and pressure would fall. 4. If the temperature of the liquid is constant, the molecules will escape from the surface at a constant rate. 5. Unless this method of study had been adopted, the students would have become engulfed in a mass of complicated phenomena from which it is quite doubtful whether they could have extricated themselves. 6. If water in an open vessel were heated, the vessel would soon become dry. 7. If liquid water is sufficiently cooled, it freezes, i. e. it changes to the solid state. 8. If this were so, fluorine gas in contact with chloride ion would remove the electron from the chloride ion and, thereby, form fluoride ion. 9. This is about half the amount of energy that would be evolved if a mole of sodium metal reacted with chlorine to form NaCL.
Ex. 7. Restate the sentences using the indirect order and translate them. Study the Model.
1. If the enthalpy change were positive, the reaction could not occur by itself. 2. If this reaction could be shown to be spontaneous, it might offer an ideal way to remove two of the most serious air pollutants, carbon monoxide and nitric oxide. 3. If we had understood the reaction mechanism, we should have been able to raise the rate of the reaction or, if necessary, to slow it down. 4. If they had investigated the properties of this substance, they would have been careful. 5. If the sodium chloride were prepared by passing hydrogen chloride gas into a dilute solution of sodium hydroxide, much heat would evolve. 6. The substance might have been identified if we had known the properties it possessed.
Date: 2015-01-29; view: 1582
|
 Chemistry has improved our quality of life, and made thousands of products possible. Unfortunately, this achievement has come at a price: our collective human health and the global environment are threatened. Our bodies are contaminated with a large number of synthetic industrial chemicals, many of which are known to be toxic and carcinogenic while others remain untested for their health effects. They come to us from unlabeled products, chemically con-taminated food, air, water and dust while the developing fetus is exposed directly to chemicals in the womb. Many chemicals work their way up the food chain and circulate round the globe: pesticides used in the tropics are commonly found in the Arctic; flame retardants used in furniture and electronics are now commonly found in marine mammals.
Chemistry has improved our quality of life, and made thousands of products possible. Unfortunately, this achievement has come at a price: our collective human health and the global environment are threatened. Our bodies are contaminated with a large number of synthetic industrial chemicals, many of which are known to be toxic and carcinogenic while others remain untested for their health effects. They come to us from unlabeled products, chemically con-taminated food, air, water and dust while the developing fetus is exposed directly to chemicals in the womb. Many chemicals work their way up the food chain and circulate round the globe: pesticides used in the tropics are commonly found in the Arctic; flame retardants used in furniture and electronics are now commonly found in marine mammals.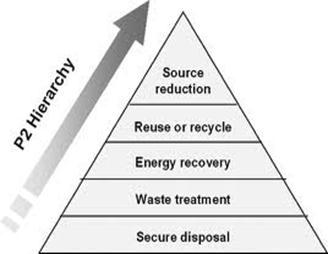 Consumers and business purchasing departments can promote green chemistry by demanding safer, non-toxic products from manufacturers. This will help to give a competitive advantage to those companies who screen the chemicals used in their products and demand safer substitutes from their suppliers. Governments have a major role in adopting policies that promote green chemistry innovation and implementation in the commercial sector. At the same time the chemical industry has a duty to integrate the principles of green chemistry into their manufacturers and retailers have a responsibility to demand chemicals from their suppliers that have been tested and shown to be inherently safe.
Consumers and business purchasing departments can promote green chemistry by demanding safer, non-toxic products from manufacturers. This will help to give a competitive advantage to those companies who screen the chemicals used in their products and demand safer substitutes from their suppliers. Governments have a major role in adopting policies that promote green chemistry innovation and implementation in the commercial sector. At the same time the chemical industry has a duty to integrate the principles of green chemistry into their manufacturers and retailers have a responsibility to demand chemicals from their suppliers that have been tested and shown to be inherently safe.