
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
STUDY OF RADIOACTIVITY PHENOMENON. DETERMINATION OF COEFFICIENT OF γ-RAYSABSORPTION BY SUBSTANCE
Task 1. Take data for laboratory work fulfilment 1.1. Take data for calculations according to the variant, given by a teacher. Enter the results into table 1. Table 1
1.2. Draw graph of the dependence using data from table 2. Table 2
1.3. Determine angle tangent of graph inclination to the x-axis. Enter the results into table 3.
1.4. Determine mass absorption factor of lead according to the formula:
Conclusions The data of laboratory work fulfilment Pass mark Signature Mark of laboratory work defence Signature
1. What does radioactivity phenomenon consist in? 2. What are the types of radioactivity? 3. Write down and formulate the radioactive decay law. 4. Give the determination of radioactive decay constant. 5. What is radioactive isotope half-life? 6. What types of radioactive decay do you know? What does displacement rule consist in? 7. Give the definition of α-, β- and γ-decays. 8. Explain the origin of α-, β-decays. 9. What is penetrability of γ-rays in a substance? 10. Write down the law of γ-rays absorption by a substance.
(theoretical information) 1. Thermal radiation. Energy characteristics of thermal radiation. Perfectly black body model. 2. Kirhhoff’s law for thermal radiation. 3. Stefan-Boltzmann law. 4. Wien’s displacement Law. 5. «Violet catastrophe». 6. Planck’s quantum hypothesis, Planck’s formula. 7. Deduce Stefan-Boltzmann law and Wien’s displacement law using Planck’s formula. 8. Thermal radiation laws application. 9. Photoelectric effect. Photoelectric effect laws. 10. Photoelectric effect threshold and stopping potential. 11. Einstein’s theory of photoelectric effect. 12. Photon and its characteristics. 13. practical application of photoeffect. 14. Light pressure. 15. Compton’s effect. 16. De Broglie’s hypothesis. 17. Experiments of Davisson and Germer. 18. Heisenberg’s principle of uncertainity. 19. Wave function and Schrodinger’s equation. 20. Free particle motion. 21. Electron in potential box. 22. Electron in potential well. 23. Tunnel effect. 24. Linear harmonic oscillator. 25. Rutherford Model of atom and radiation problem. 26. Bohr’s postulates. 27. Balmer’s series formula. 28. Franck and Hertz experiments. 29. Physical quantities quantization: energy, momentum, angular momentum, space quantization. 30. Quantum numbers. Pauli’s principle. Electrons distribution by their states. 31. Spectrums of multielectronic atoms. Zeeman’s Effect. Characteristic X-ray spectra. 32. Spontaneous and forced radiation. Quantum generators. 33. Composition and properties of nucleons. 34. Atomic nucleus properties. Energy levels. 35. Atomic nucleus quantum descriptions. 36. Fundamental interaction in nature. 37. Nuclear forces properties. Virtual pion. 38. Nucleus binding energy. Specific binding energy. 39. Nucleus models. Characteristics. 40. Radioactive decay. Law of radioactive transformation (deducing). 41. Half-life, activity. 42. Alpha decay. Peculiarities, characteristics. 43. Beta-decay. Peculiarities, characteristics. 44. Gamma-decay. Peculiarities, characteristics. 45. Nuclear reactions. Types and features of nuclear reactions. 46. Nuclear reaction effective cross section. 47. Methods of nuclear reactions recording. Nuclear reaction energy. 48. Elementary particles. Quantum characteristics and mutual transformations of elementary particles. 49. Conservation laws. 50. Elementary particles classification. 51. Quarks: classification and characteristics. 52. Quarks interaction
Date: 2015-01-12; view: 1016
|
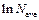
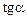
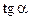 = ___________ .
= ___________ .
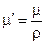 .
. questions to be admitted for doing laboratory work
questions to be admitted for doing laboratory work QUESTIONS FOR CURRENT TEST PAPERS AND MODULE CONTROL
QUESTIONS FOR CURRENT TEST PAPERS AND MODULE CONTROL