
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Chemical Properties
The alkali metals are strong reducing agents. The standard electrode potentials all lie between -2,7V and -3,0V, indicating a strong tendency to form cations in solution. They can reduce Oxygen, Chlorine, Ammonia and Hydrogen. The reaction with Oxygen tarnishes the metals in air, so they are stored under oil. They cannot be stored under water because they react with it to produce Hydrogen and alkali hydroxides:
2M + 2H2O = 2MOH + H2.
This reaction illustrates the increasing reactivity on descending the Group. Li reacts steadily with water, with effervescence; Sodium reacts more violently and can burn with an orange flame; K ignites on contact with water and burns with a lilac flame; Cs sinks in water, and the rapid generation of Hydrogen gas under water produces a shock wave that can shatter a glass container. Na dissolves in liquid Ammonia to give a deep blue solution of Sodium cations and solvated electrons. This solution is used as a reducing agent.
At higher concentrations the color of the solution changes to bronze and it conducts electricity like a metal.
The chemistry of Li shows some anomalies, as the cation Li+ is so small it polarizes anions and so introduces a covalent character to its compounds. Li has a diagonal relationship with Magnesium.
Oxides
The Alkali metals form ionic solid oxides of composition M2O when burnt in air. However, Na also forms the peroxide Na2O2 as the main product, and K forms the superoxide KO2, also as the main product:
4Li + O2 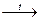 2Li2O;
2Li2O;
2Na + O2 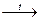 Na2O2;
Na2O2;
K + O2 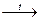 KO2.
KO2.
Hydroxides
Alkali metal hydroxides are white ionic crystalline solids of formula MOH, and are soluble in water. They are all deliquescent except LiOH. The aqueous solutions are all strongly alkaline (hence the name of this Group) and therefore dangerous to handle. They neutralize acids to form salts, eg: NaOH + HCl= NaCl + H2O.
Halides
Alkali metal halides are white ionic crystalline solids. They are all soluble in water except LiF.
Oxidation States
Alkali metals have oxidation states of 0 and +1. All the common compounds are based on the M+ ion. This is because the first ionization energy of these elements is low, and the second ionization energy is much higher. The outermost electron is well shielded from the attraction of the nucleus by filled inner electron levels and so is relatively easy to remove. The next electron is much more difficult to remove as it is part of a full level and is also closer to the nucleus.
Industrial Information
Sodium hydroxide, Chloride and Carbonate are among the most important industrial chemicals associated with this Group. Sodium hydroxide is produced by the electrolysis of saturated brine in a cell with steel cathodes and titanium anodes. Sodium Carbonateis made by the Solvay Process, in which soluble Sodium Chloride is converted into insoluble Sodium Hydrogen Carbonate and filtered off, then heated to produce the Carbonate. However, the principal by-product of this process is calcium Chloride, and its deposition in rivers causes environmental concern. The Solvay Process is therefore gradually being replaced by the purification of Sodium Carbonate from minerals.
Date: 2015-01-12; view: 912
| <== previous page | | | next page ==> |
| Appearance | | | Other bioactive metals |