
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Experiment 5. The salts of hydrohalogen acids insoluble in waterPut 3-5 drops of solutions hydrochloric and hydroiodic acids salts (for example, NaCl and KI) into the test-tubes and add into each several drops of Pb(NO3)2. Determine the color of occurred precipitates, write the equations.
CHAPTER # 12. THE CHALCOGENS
1. Introduction in General, Organic and Biochemistry, 7th Edition, by Morris Hein, Leo R. Best, Scott Pattison and Susan Arena, Brooks/Cole Publishing Co., 2001. (Chapter 10, pp. 289-294, Chapter 27, pp. 765-777); 2. http://www.chemsoc.org/viselements/pages/Visual Elements Group 16.html 3. http://www.hrw.com/science/mc/index/htm
1. General characteristics
The elements of Group 6A (or the main sub-group of VI group) are:
Often this group of chemical elements (except the last Po) is named the Chalcogens. Appearance The first element of this sub-group, Oxygen, is the only gas, and is colorless and odorless. Sulfur is a pale yellow, brittle solid. Selenium can have either an amorphous or a crystalline structure; the amorphous form can be red or black, and the crystalline form can be red or gray. Tellurium is a silvery-white color with a metallic luster. Polonium is a naturally radioactive element. Selenium and Tellurium are rare elements with few uses, and along with Polonium will not be considered further here.
General Reactivity Oxygen and Sulfur are highly electronegative elements - the electronegativity of Oxygen is second only to that of Fluorine. Their general reactivity is therefore dominated by their ability to gain electrons. There is a transition down the sub-group from non-metallic to more metallic properties, so that Oxygen is a non-metal and Tellurium a metalloid. All the elements except Polonium form M2- ions. There is a marked difference between Oxygen and the other members of the Group. This arises from: Ø the small size of the O atom which enables it to form double bonds; Ø its inability to expand its valence shell like the other elements as it has no accessible d-orbitals; Ø its high electronegativity, which enables it to participate in Hydrogen-bonding. Occurrence and Extraction Oxygen occurs widely as the free element in the form of O2, comprising 21% of the air by volume. It also occurs as O3, ozone, at high altitudes in the ozone layer. In the combined form it is found in very many minerals, and also in water. Oxygen is obtained industrially by the fractional distillation of liquid air. It is stored under pressure in cylinders. Sulfur is found as the free element and also as metal Sulfide ores and a number of Sulfates. Native sulfur is brought to the surface from underground deposits by the Frasch Process, which uses superheated water to melt the Sulfur and force it upwards. Physical Properties The covalent and ionic radii increase going down the sub-group, as electrons occupy shells with higher quantum numbers. Oxygen occurs as two gaseous allotropes, O2 (dioxygen or more commonly Oxygen) and O3 (trioxygen or ozone). Oxygen is more common. It condenses to a pale blue paramagnetic liquid at -1830C. Ozone is a pale blue, pungent gas, which condenses to an inky-blue liquid at -1120C. The ozone layer in the upper atmosphere is an important shield against harmful UV radiation from the Sun. Sulfur has several allotropes, the two main ones being rhombic and monoclinic sulfur. These both consist of S8 molecules. Industrial Information Sulfuric acid is of immense industrial importance. Because it has three chemical functions and is very cheap to produce, Sulfuric acid is used at some stage of the manufacture of most products. It is said that the economic prosperity of a country can be assessed by its consumption of sulfuric acid. It is manufactured by the Contact Process. Hydrogen peroxide H2O2 is used to bleach hair and textiles, as a mild disinfectant and in pollution control. Sulfur hexafluoride SF6 cannot be ionized by electric fields and so is widely used as a gaseous insulator in transformers and electrical switchgear. Oxygen
Oxygen is the element of the second period, its formula is 1s22s22p4.
On the assumption of external electron shell structure (graphic presentation) and electronegativity of Oxygen, it is characterized by oxidation number equal to 2-, moreover, in composition with Fluorine the oxidation number of Oxygen will be 2+ (for example, OF2) but in peroxides it will be 1- (for example, H2O2) or, sometimes, -½ (for example, superoxide KO2). Oxygen, like Fluorine, forms compounds with all elements except Helium, Neon and Argon. Atomic Oxygen is extremely active. In elemental state Oxygen is mostly characterized as the oxidizing agent in the reactions: 1) 4FeS + 7O2 = 2Fe2O3 + 4SO2
2) 2 KI + O3 + H2O = I2 + 2 KOH + O2
Oxygen compounds in which the oxidation number is equal to 1- are called peroxides. For example, Hydrogen peroxide H2O2, where covalent and non-polar bonds in molecule connect two atoms of Oxygen. Water solution of Hydrogen peroxide (perhydrol) presents weak dibasic acid: H2O2 ↔ H+ + HO2-; HO2- ↔ H+ + O22-. Medium of oxidation number of Oxygen in peroxides causes its oxidation-reduction duality in the reactions. H2O2 is the reducing agent: H2O2 + 2KMnO4 + 2KOH = 2K2MnO4 + 2H2O + O2
but in presence of strong reducing agent it is oxidizing agent: H2O2 + 2KI + H2SO4 = I2 + K2SO4 + 2H2O
Ozone is also a highly reactive and powerful oxidizing agent which can cleave the C=C double bond.
Sulfur
In contrast to Oxygen, Sulfur atom has free d-sublevel in the external electronic shell: 1s22s22p63s23p43d0. Accordingly, Sulfur can form the compounds due to two, four and six unpaired electrons, showing such oxidation levels: 2-, 0, 2+, 4+, 6+: Ground (nonexcited) state ( 2-, 0, 2+ oxidation number, for example, in H2S, S, SO compounds):
The first excited state (4+ oxidation number, for example, in SO2 compound):
The second excited state ( 6+ oxidation number, for example, in H2SO4 compound):
Characteristic of the most important sulfur compounds is shown in Table 16.
Table 16. Characteristic of the most important Sulfur compounds
Sulfur is the typical non-metal, which is inferior to Halogens, Oxygen and Nitrogen in electroactivity and thus is oxidized by them: S + O2 = SO2; S + 2Cl2 = SCl4. As oxidizing agent, Sulfur reacts with the metals and Hydrogen at high temperature. For example, Zn + S = ZnS; S + H2 = H2S. Hydrogen sulphide (H2S) is the gas and occurs in nature as the result of putrescent bacteria action to proteins containing sulfur so it can be detected by its characteristic odor (“bad eggs gas”). Inhalation of pure Hydrogen sulphide can bring about to immediate death; even 0,01% of its concentration in the air is extremely dangerous for human being. Aqueous solution of H2S is the weak dibasic acid; therefore there are acid and neutral salts (sulphides and hydrosulphides): H2S ↔ H+ + HS-; HS- ↔ H+ + S2-. H2S is strong reducing agent. In air it burns with the generation of SO2 or S (in case of Oxygen deficit): 2 H2S + 3 O2 = 2 H2O + 2 SO3
or 2 H2S + O2 (in deficit) = 2 H2O + 2 S
Sulfur dioxide (SO2) is sulfurous gas (pungent, choking gas) formed when Sulfur or Sulfides are burnt in air or Oxygen, and as all fossil fuels contain Sulfur it is formed when they burn and contributes to the problem of “acid rain” : S + O2 2CuS + 3O2 4FeS2 + 11O2 = 2Fe2O3 + 8SO2↑. SO2 may be prepared by Sulfuric acid reduction: 2H2SO4 (conc) + Cu = CuSO4 + SO2 ↑ + 2H2O. Aqueous solution of SO2 is called Sulfurous acid, which is unknown in free state. H2SO3 is of medium strength and can produce acid and neutral salts. Sulfurous acid salts decay in case of strong acids action with SO2 isolation. This is the base for getting of Sulfur dioxide in laboratory conditions: Na2SO3 + 2HCl = 2NaCl + SO2 ↑ + H2O. The medium of oxidation number in Sulfur (IV) compounds causes their oxidation-reduction duality: Na2SO3 + Cl2 + H2O = NaHSO4 + HCl + NaCl
SO2 + 2 H2S = 3 S + 2H2O
SO2 is used for fumigation of basements, cellars in order to avoid funguses. Calcium hydroSulfite Ca(HSO3)2 with sulfurous acid is used for sulfitation - one of the methods for preserving of tender fruits and vegetables. Sulfur trioxide (SO3) or Sulfur (VI) oxide is the Sulfur acid anhydride. In industry it is produced by SO2 oxidation in presence of catalyst (Pt) (in so-called Contact Process): 2 SO2 + O2 = 2 SO3. Sulfuric acid is manufactured as the result of SO2 interaction with water or dilute sulfuric acid: SO3 + H2O = H2SO4. Strong acidic properties of H2SO4 solutions are the basis for their use in fertilizer production or mineral premixes for animals (simple and triple superphosphates, Ammonium Sulfate): Ca3(PO4)2 +2H2SO4 + 5H2O = Ca(H2PO4)2·H2O + 2[CaSO4·2H2O];
Simple superphosphate
Ca3(PO4)2 + 3H2SO4 = 2H3PO4 + 3CaSO4;
Triple superphosphate H2SO4 + 2NH3 = (NH4)2SO4. Concentrated H2SO4 is a fairly strong oxidizing agent. Non-metals are oxidized by it up to oxides and H2SO4 itself reduces to SO2:
C + 2 H2SO4 (conc) = CO2 + SO2 + 2 H2O
PRACTICE PROBLEMS 1. Detect Oxygen and Sulfur oxidation numbers in the following compounds: O2, O3, CaO, BaO, OF2, S8, FeS, S2, K2SO4, Na2SO3, SO3, Al2(SO4)3. 2. At which numbers of oxidation does Sulfur present oxidation-reduction duality? 3. In which cases does Oxygen present the properties of reducing agent? 4. Why Hydrogen Sulfide cannot act as the oxidizing agent? 5. Set up the reaction of Sulfuric acid getting taking into account the following series of conversions: FeS2 ® SO2 ® H2SO3 ® H2SO4. 6. Set up the reaction according to series of conversions: SO3 ® H2SO4 ® (NH4)2SO4 ® CaSO4. 7. Complete the equations of redox reactions: H2S + H2O2 ® HClO + H2O2 ® KMnO4 S + NaOH ® Na2S + Na2SO3 + … NaHSO3 + Cl2 + H2O ® NaHSO4 + …
LABORATORY TRAINING
Experiment 1. Oxidizing properties of H2O2 Add 2-3 drops of H2SO4 and 3-4 drops of Hydrogen Peroxide to 2-3 drops of Potassium or Sodium iodide. Prove free Iodine release in given reaction by adding starch solution. Set up the reaction.
Experiment 2. Reducing properties of H2O2 Drop H2O2 up to discoloration of the mixture to the test-tube to 2-3 drops of KMnO4, acidified by 1-2 drops of dilute H2SO4. What gas is released? Set up the reaction.
Experiment 3. Reducing properties of S2- Fill test-tube with 5-7 drops of hydroSulfide water and add some drops of chloric water up to mixture turbidity. Fill another test-tube with 5-7 drops of Sodium or Ammonium Sulfide. Add 3-4 drops of concentrated nitric acid and watch solution turbidity as the result of sulfur generation. Set up the reactions.
Experiment 4. Reducing properties of S4+ Fill test-tube with 5-7 drops of Na2SO3, acidify by 3-4 drops of H2SO4 and add 3-5 drops of iodic water (acting component I2). Why does the color of I2 disappear? Write the reaction.
Experiment 5. Oxidizing properties of S4+ Acidify 5-6 drops of Na2SO3 by 2-3 drops of diluted H2SO4 and add some drops of hydrosulfuric water up to mixture turbidity. Write the reaction.
Date: 2015-01-12; view: 846
|
 ® 2O2-
® 2O2-
 4 (oxidation).
4 (oxidation).
 Strengthening of acid properties
Strengthening of acid properties
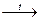 SO2 ↑;
SO2 ↑;
 Ca3(PO4)2 + 4H3PO4 + 3H2O = 3 Ca(H2PO4)2·H2O.
Ca3(PO4)2 + 4H3PO4 + 3H2O = 3 Ca(H2PO4)2·H2O.