
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
LABORATORY TRAINING
Experiment 1. Compound formation with complex cation
Add by drops ammonia solution firstly up to the formation of HydroxoSulfate and then up to its complete dilution and formation of complex compound [Cu(NH3)4]SO4 into the test-tube with 4-5 drops of cuprous Sulfate or Chloride (II). How does the initial color of the solution change? Equate the reaction according to the process stages of reagent interactions.
Experiment 2. Compound formation with complex anion
A) Put 4-5 drops of Hg(NO3)2 solution into the test-tube and add by one drop of KI solution up to the formation of red precipitate of Mercury (II) iodide. Continue to add KI solution up to complete dilution of precipitate and formation of soluble complex compound - Potassium Tetraiodomercurate (II). Equate the reaction taking into account the stages.
B) Put 5-7 drops of Cobalt (II) Sulfate or Chloride solution into the test-tube. Add little by little the granules of KNO2 up to solution saturation (there are some undissolved salt at the bottom of test-tube). Add by drops concentrated acetic acid to the mixture up to the formation of yellow precipitate of K3[Co(NO2)6]. Equate the reaction according to stages taking into account that nitrous acid, which is isolated under acidation, oxidizes Co2+ to Co3+:
CoCl2 + KNO2 →
KNO2 + CH3COOH → HNO2 +…
Co(NO2)2 + HNO2 → NO +…
Co(NO2)3 + KNO2 →
Experiment 3. Complex compounds in metathesis
A) Add 2-3 drops of K3[Fe(CN)6] solution to 4-5 drops of any Iron (II) salt solution. What is the color of precipitate? Equate molecular and ionic reactions.
B) Add 2-3 drops of K4[Fe(CN)6] solution to 4-5 drops of any Cooper (II) salt solution. Identify the color of precipitate and equate molecular and ionic reactions.
C) Add 2-3 drops of K4[Fe(CN)6] solution to 4-5 drops of Iron (III) salt solution. Identify the color of precipitate and equate molecular and ionic reactions.
D) Add 2-3 drops of KSCN or NH4SCN solution to 4-5 drops of Iron (III) salt solution. Identify the color of precipitate and equate molecular and ionic reactions.
CHAPTER # 11. THE HALOGENS
1. Introduction in General, Organic and Biochemistry, 7th Edition, by Morris Hein, Leo R. Best, Scott Pattison and Susan Arena, Brooks/Cole Publishing Co., 2001. (Chapter 28, pp. 779-794);
2. http://www.chemsoc.org/viselements/pages/Visual Elements Group 17 - The Halogens.html
3. http://www.hrw.com/science/mc/index/htm
1. General characteristics
The elements of Group 7A (or the main sub-group of VII group), the Halogens, are:
| Symbol | Electron configuration |
| Fluorine F | [He]2s22p5 |
| Chlorine Cl | [Ne]3s23p5 |
| Bromine Br | [Ar]4s24p5 |
| Iodine I | [Kr]5s25p5 |
| Astatine As | [Xe]6s2 6p5 |
All the isotopes of astatine are radioactive, and so this element will not be considered further here. The similarity of their properties is conditioned by similar electronic structure of the atoms where the external energy level is expressed by the electronic formula ns2 np5. Moreover only F has no d-sublevel and as the result it is exclusively one-valent. Taking into account that F is the most electronegative element it is characterized by two levels of oxidation in compounds (0 and 1-).
Appearance
Fluorine is a poisonous pale yellow gas, Chlorine is a poisonous pale green gas, Bromine is a toxic and caustic brown volatile liquid, and Iodine is a shiny black solid, which easily sublimes to form a violet vapor on heating.
General Reactivity
The elements of Group 17, the Halogens, are very similar set of non-metals. They all exist as diatomic molecules, X2, and oxidize metals to form halides. The halogen oxides are acidic, and the hydrides HX are covalent. Fluorine is the most electronegative element of all. Generally, electronegativity and oxidizing ability decrease on descending the Group. The result of this decreasing electronegativity is increased covalent character in the compounds, so that AlF3 is ionic whereas AlCl3 is covalent.
Fluorine shows some anomalies because of the small size of its atom and ion. This allows several F atoms to pack around a different central atom, as in [AlF6]3- compared with [AlCl4]-. The F-F bond is also unexpectedly weak because the small size of the F atom brings the lone pairs closer together than in other halogens, and repulsion weakens the bond.
Occurrence and Extraction
The Halogens are too reactive to occur free in nature. Fluorine is mined as fluorspar, calcium fluoride and cryolite. It is extracted by electrolysis as no oxidant will oxidize fluorides to Fluorine. Chlorine is also found in minerals such as rock salt, and huge quantities of Chloride ions occur in seawater, inland lakes and subterranean brine wells. It is obtained by the electrolysis of molten Sodium Chloride or brine. Bromine is also found as the bromide ion in seawater, and in larger quantities in brine wells, from which it is extracted. Iodine is mined as Sodium Iodate (V) NaIO3, which is present in Chile saltpetre. It is obtained by reaction with Sodium Hydrogen Sulfite.
Physical Properties
At room temperature all the Halogens exist as diatomic molecules. The melting points, boiling points, atomic radii and ionic radii all increase on descending the Group. More than 8 electrons never surround Fluorine, whereas the other halogens may be surrounded by up to 14 electrons.
Chemical Properties
The most characteristic chemical feature of the Halogens is their ability to oxidize. Fluorine has the strongest oxidizing ability, so other elements, which are combined with Fluorine, have their highest possible oxidation number:
2F2 + H2O = 2HF + OF2
F20 + 2  ® 2F- ® 2F-
| 2 (reduction); |
O2- - 4  ® O2+ ® O2+
| 1 (oxidation). |
2F2 + 2NaOH ® 2NaF + OF2 + H2O.
Fluorine is such a strong oxidizing agent that it must be prepared by electrolysis of solid salts:
2NaFsolid 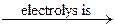 2Na + F2↑.
2Na + F2↑.
Chlorine is the next strongest oxidizing agent, but it can be prepared by chemical oxidation. Most elements react directly with Chlorine, Bromine and Iodine, with decreasing reactivity going down the Group, but often the reaction must be activated by heat or UV light. The oxidation of Thiosulfate ions, S2O32-, by the Halogens is quantitative.
This means that oxidizing agents can be estimated accurately; the oxidizing agent is reacted with excess I- ions, and the liberated I2 titrated with standard Thiosulfate solution. The end point is detected with starch as indicator, which forms a dark blue complex with Iodine.
Chlorine, Bromine and Iodine disproportionate in the presence of water and alkalis.
Oxides and Oxoacids
There are no Fluorine oxides as F is more electronegative than O. Chlorine, Bromine and Iodine each form several oxides, which are thermally unstable, such as Chlorine dioxide ClO2. The only Fluorine oxoacid, HOF, is unstable at room temperature, but there are many oxoacids of the other Halogens. The best-known salts of these are: Hypochlorite, Chlorate (I) CIO-, Chlorite, Chlorate (III) ClO2-, Hypochlorate, Chlorate (V) CIO3-, and Perchlorate, Chlorate (VII) ClO4-. These are all powerful oxidizing agents.
Halides
The Halogens can be combined with each other to form interhalogens and polyhalide ions. Olyhalide ions have the general formula [Y-X-Y]-. It is not possible for F to represent X in a polyhalide ion, as it cannot expand its octet.
Hydrides
Hydrogen halides have the general formula HX. HF is a colorless liquid, which boils at 19,50C, and all the other Hydrogen halides are colorless gases. HF is a liquid due to the extensive Hydrogen bonding which occurs between molecules. All the Hydrogen halides are easily dissolved to give acidic solutions, the most widely used being hydrochloric acid, HCl. All except HF are typical acids; they liberate carbon dioxide from Carbonates and form salts with basic oxides. HF is a weak acid because the H-F bond is very strong, and because Hydrogen-bonding occurs between F- and HF in solution.
Oxidation States
Fluorine in all its compounds has an assigned oxidation number of -1, as it is the most electronegative of all the elements. The other halogens show a wide range of oxidation numbers, and the redox chemistry of these halogens is important. The oxidation numbers most commonly shown are odd; there are few compounds with even oxidation numbers and they are often thermally unstable. Chlorine is the third most electronegative element after F and O. The halide ions are readily formed by accepting one electron, as this completes an octet of valence electrons. The electron affinity decreases on descending the Group.
Industrial information
The Halogens are probably the most important Group of the Periodic Table used in industry. Fluorine is widely used as an oxidizing agent. HF is used to etch glass. Chlorine is used for chlorinating of drinking water, and in many organochlorine compounds. Some of these, such as the insecticide DDT, are effective but environmentally damaging, and much controversy surrounds their use. Chlorine dioxide ClO2 is used to bleach wood pulp for paper making, as it gives a good whiteness without degrading the paper. Hypochlorites are used in domestic bleaches. Potassium chlorate (V) KClO3 is used as an oxidant in fireworks and matches. The properties of the Halogens on the example of Chlorine will be presented below.
Date: 2015-01-12; view: 1085
| <== previous page | | | next page ==> |
| Rules for naming of coordination compounds | | | Chlorine |