
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
HYDROLYSIS OF SALTS
1. Introduction in General, Organic and Biochemistry, 7th Edition, by Morris Hein, Leo R. Best, Scott Pattison and Susan Arena, Brooks/Cole Publishing Co., 2001. (Chapter 19.3, pp. 580-583);
2. http://antoine.frostburg.edu/chem/senese/101/acidbase/fag/hydrolysis/
3. http://www.chemtutor.com/acid.htm#salts
1. Ionic product of water. Notion of pH
When the law of mass action is applied to the dissociation of water, we have:
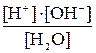 =K or [H+]·[OH-] = K·[H2O].
=K or [H+]·[OH-] = K·[H2O].
Denoting K·[H2O] by Kw, we obtain [H+]·[OH-] = Kw. The quantity Kw is equal to product of concentration of the Hydrogen and hydroxyl ions in water, and, is, therefore, called the ionic product of water. Its numerical value can easily be found, since K and [H2O] are known: the dissociation constant of water, K, is equal to 1,8·10-16, while the concentration of undissociated water molecules is, owing to insignificant dissociation, practically equal to the total number of moles of water per litre, i.e. 100/18=55,56 moles/L. Consequently, Kw= 1,8·10-16 x 55,56 = 1·10-14.
The ion product of water is an extremely important value, since it enables the concentration of OH-ions to be found for any aqueous solution with a known concentration of H+ ions and vice versa. For example, for pure water
[H+] = [OH-] =  =10-7 g-ions/L.
=10-7 g-ions/L.
By using the ion product of water, the acidity or alkalinity of any solution can be expressed in terms of Hydrogen ion concentration (Fig. 16).
| [H+] | 10-2 | 10-3 | 10-4 | 10-5 | 10-6 | 10-7 | 10-8 | 10-9 | 10-10 | 10-11 | 10-12 | [H+] |
| ← Increasing acidity | Increasing alkalinity → | |||||||||||
| pH | pH |
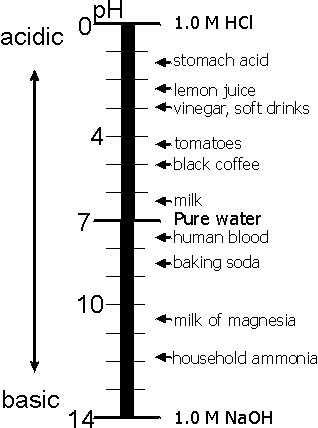
Figure 16. Acidity and alkalinity scale
In neutral solution, [H+] (often denoted by CH) is, according to the foregoing, equal to 10-7. Obviously, it is higher in an acid solution and lower in an alkaline. Thus, as  the solution becomes more and more acid, ŃH will pass through the values 10-6, 10-5, 10-4, etc. And, conversely, as the solution becomes more and more alkaline, we shall have: 10-8, 10-9, 10-10, etc.
the solution becomes more and more acid, ŃH will pass through the values 10-6, 10-5, 10-4, etc. And, conversely, as the solution becomes more and more alkaline, we shall have: 10-8, 10-9, 10-10, etc.
The quantitative designation of the acidity or alkalinity of a solution may be still further simplified by using the Hydrogen ion concentration index (pH). The relation defines this index:
pH = - lg CH+ - negative common logarithm of H+-ions concentration (more punctually - H+-ions activity). Thus a neutral solution will have pH=7, an acid solution will have pH less than 7, and an alkaline solution will have pH more than 7.
In practice, the acidity or alkalinity of a solution is conveniently determined by means of indicators – substances that change color depending on the relative concentrations of H+ and OH- ions. The best-known indicator is litmus, which turns red when there is a surplus of H+ ions, i.e., in acid solution, blue when there is a surplus of OH- ions, i.e., in alkaline solution, and violet in neutral solution. If filter paper impregnated with litmus (“litmus paper”) is immersed in the solution to be tested, its color shows at once whether the solution is acid, alkaline or neutral.
The color change of different indicators occurs at different Hydrogen ion concentrations, which is important for chemical analysis. For example, litmus changes its color (red to blue) at approximately pH=7, methyl orange (red to yellow) at pH=4, and phenolphthalein (colorless to pink) at pH=9.
For more precious measuring of pH it is widely used the special tools – pH-meters, which provides assurance of measuring within the limits of ± 0,01.
Date: 2015-01-12; view: 1107
| <== previous page | | | next page ==> |
| Ionic equations | | | General notion of Hydrolysis |