CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
PRACTICE PROBLEMS1. What are the three major types of bonds? 2. What is the role of electronegativity in the determination of the ionic or covalent character of a bond? 3. What type of bond would be expected between H and F; Cu and S; Br and I? 4. List the three bond pairs referred to in the previous question in order to increase ionic characters.
—HAPTER # 5. LABORATORY GLASSWARE, LABWARE AND RULES OF LABORATORY RESEARCH
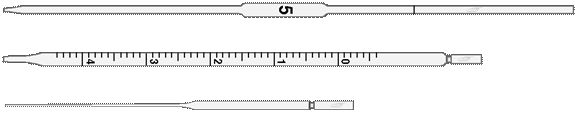
The most useful chemical glassware is presented in Fig. 12 and 13.
Spatulas
Volumetric pipettes
Crystallizing Dish as sand bath
Figure 13. Separate varieties of laboratory glassware
1. Chemical glassware
All chemical glassware is divided into several groups according to their use: Ø of general usage (is used to carry out different chemical operations): test tubes, beakers, flat-bottom, round-bottom and conical flasks, Wurtz flasks (round-bottom with bleeder), crystallizer tanks, funnels, clock glass, weighing bottles; Ø measuring vessels: graduated cylinders, measuring tubes, measuring pipettes, volumetric flasks; Ø of special use: flasks, Kipp gas generator, vacuum filtering apparatus, consisting of Bunsen flask, Buchner funnel, trap flask, and water-jet pump; Ø porcelain ware and ware made from other materials: spreading rods, mortars, evaporating dishes, crucibles and boats.
Date: 2015-01-12; view: 1210 |

 Figure 12. Chemical Glassware
Figure 12. Chemical Glassware