CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
The Periodic Table
The Periodic Table is an arrangement of the elements according to their atomic numbers so that elements with similar properties fall in the same column. The length of each period in the Periodic table determinates by the sublevel being filled with electrons as shown in table 7. The first energy level holds only two electrons in its 1s sublevel. Hydrogen and Helium are presented in the first period. The second main-energy level holds two electrons in 2s-sublevel and six electrons in 2p-sublevel. These eight electrons account for the eight elements presence in the second period. In eight elements of the third period, 3s- and 3p-sublevels are being filled. Table 7. Relationship between quantity of elements in period and sublevels being filled in the Periodic Table
Filling 3d and 4d-sublevels in addition to s and p sublevels adds 10 elements to fourth and fifth periods, which therefore include totally 18 elements each. Filling 4f sublevels in addition to s, p, d sublevels adds 14 elements to the sixth period, for totally of 32 elements in the sixth period. As new artificial elements are created, the 23 known elements in period 7 could be extended to 32. The period of element can be determined from its electron configuration. For example, Arsenic (As) has the configuration [Ar]3d104s24p3. The “4” in “4p3” indicates that the highest one occupied energy level is the fourth energy level. Arsenic is therefore in the fourth period in the Periodic Table. There are a lot of styles for presentation of Periodical Table of Chemical Elements (authors Stowe, Benfey, Zmaczynski, Gicuere - more details http:/chemlab.pc.maricopa.edu/periodic/styles.html). In Ukraine the most popular one is so-called “Short-Periodic” form, presented in Appendix 1. The Modern style of so-called “Long-Periodic” table is presented in Fig. 4.
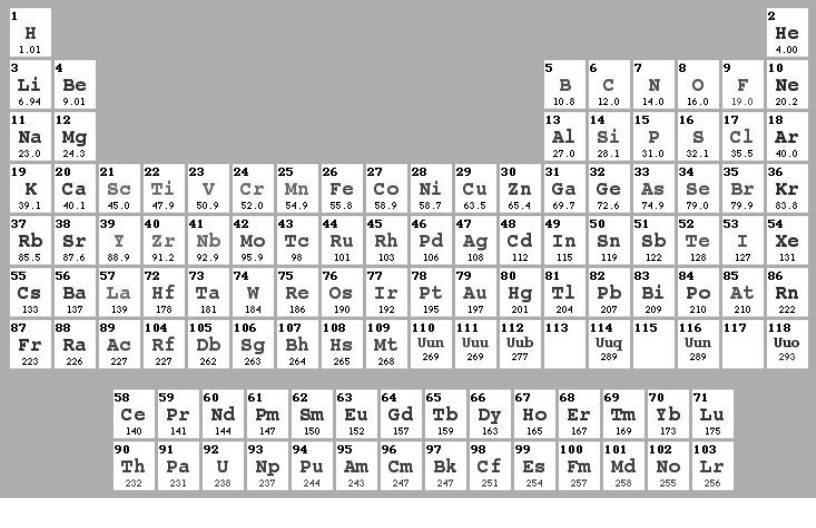
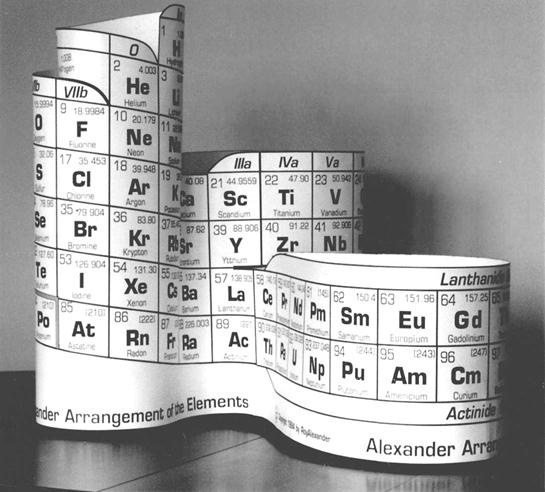
Figure 4. “Long-Periodic” style of Periodic table A science exhibit designer, Roy Alexander, to eliminate the confusion and apparent inconsistencies in the flat table by arranging the elements contiguously and continuously according to the atomic number without disturbing the accepted group, originated the improvement for learning and using the Periodic Table and property interrelationships previously found in the periodic chart (see Fig. 5).
Figure 5. Roy Alexander Arrangement of the Elements
The resulting form has three parts looping outwards from a relatively central point. The elements in Groups 0 (VIII), Ia, IIa, IIIb, IVb, Vb, and VIIIb are in the narrowest and tallest part, which forms a tube in the upper part, topped by a “crown” of Hydrogen. From the lower part of this component the sides branch to a lengthier loop showing IIIa, IVa, Va, VIa, VIIa, VIII, Ib, and IIb groups. From the lower half of this, the third, and longest loop protrudes the f-block. Printed as a flat sheet, it can be easily assembled into a 3-D model by teacher or student. All printed element data, therefore, have a common plane so their electron numbers can trace the elements without changing direction or leaving the surface. Date: 2015-01-12; view: 2769
|