CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Figure 2. Scheme of Electron orbital
Motion of electrons around nucleus is described by energy and structure of atomic orbital. State of electron is described by values of 4 quantum figures (Tables 3, 4).
Table 3. Names and physical content of quantum figures
Table 4. Schematic description of atomic orbital
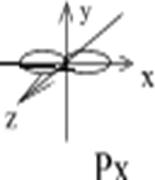
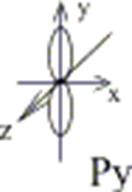
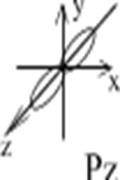
Example: To show a p-orbital: l=1, ml= -1; 0; +1; p-orbital has 3 variants of space orientation:
Figure 3. Spatial orientation of p-orbitals
Date: 2015-01-12; view: 975
|