
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
The laws and concepts of Stoichiometry
Stoichiometry is the branch of chemistry dealing with mass relationships of elements in compounds and among reactants and products in chemical reactions. Accordingly, the calculations of the quantitative relationships between elements in compounds or between substances in chemical reactions are called stoichiometric calculations. They are based on the laws of conservation of mass, of definite proportions, of multiple proportions, and also the gas laws - Gay-Lussac's law of combining volumes and the Avogadro’s law. The laws listed below are customarily considered to be the fundamental laws of stoichiometry.
Situation differs in the case of nuclear reactions. Nuclear reactions involve changes in the nuclei of atoms whereas chemical reactions involve only the outermost electrons of atoms. Another significant difference between the two types of reactions is in the amount of energy liberated. Nuclear reactions involve energies a million times greater than those of chemical reactions. Due to this a significant amounts of matter are transformed into energy in a nuclear explosion or reaction. The quantitative composition of many substances was studied from the end of the 18th century. This led to the establishment of the law of definite proportions (also called the law of constant composition) by a Frenchman, Joseph Proust, in 1797: The masses of the elements forming a given compound are always in a definite proportion that does not depend on how this compound was prepared. Many elements in combining with one another can form different substances each of which is characterized by a definite proportion of the masses of these elements. For instance, Nitrogen forms five compounds with Oxygen, Table 1. For example, one of them - Nitrogen monoxide - always contains 46,7% (mass) of Nitrogen and 53,3°/o (mass) of Oxygen. In studying such compounds, J. Dalton in 1803 established the law of multiple proportions: If two elements form several chemical compounds with each other, then the masses of one of the elements corresponding to the same mass of the other element in these compounds are in a simple integral proportion. Table 1. Mass ratio between elements in Nitrogen oxides
The laws of definite proportions and multiple proportions follow from the atomic and molecular concepts. Substances having a molecular structure consist of identical molecules. When several compounds are formed from two elements, the atoms of these elements combine with one another into molecules of a different, but definite composition, see Table 1. Unlike the law of conservation of mass, whose correctness was completely confirmed by the discoveries made after its establishment, the laws of definite proportions and multiple proportions were found to be not so universal. In connection with the discovery of isotopes, it was found that the ratio of the masses of the elements forming a given substance is constant only provided that the isotopic composition of these elements is constant. A change in the isotopic composition of an element is attended by a change in the mass composition of the compound. The laws of definite proportions (the law of constant composition) and multiple proportions were found to be not realizing also for the compounds of varying composition (berthollides, named in honor of understanding chemist Bertolle). In these compounds, for each unit of mass of a given element there may be a varying mass of another element. For example, in a compound formed by Bismuth with Thallium (metal alloy), there may be from 1,24 to 1,82 units of mass of Bismuth per unit of mass of Thallium (TlBi1,24-1,82). Compounds of varying composition are encountered also among other solids, for example oxides (UO2,5-3,0, FeO0,85-0,95), compounds of metals with Sulfur, Nitrogen, Carbon and Hydrogen (TiH0,88-1,00). Compounds of varying composition have an atomic structure instead of a molecular one, the formula only reflect the limits of the composition of the relevant substance. For many compounds of varying composition, the limits within which their composition may change have been found. So, a conclusion can be made that compounds with molecular structure always have constant composition, otherwise the composition of compounds may change in some range (homogeneity area) that depend on conditions of its obtaining. It follows from the law of definite proportions that elements combine with one another in strictly definite quantitative proportions. This is why the concepts of equivalent and equivalent mass were introduced in chemistry. At present an equivalent of an element is defined to be an amount of it such that combines with one mole of Hydrogen atoms or replaces the same number of Hydrogen atoms in chemical reactions. For example, in the compounds HC1, H2S, NH3, and CH4, the equivalents of Chlorine, Sulfur, Nitrogen, and Carbon are 1, 1/2, 1/3, and 1/4 mole, respectively. The mass of one equivalent of an element is called its equivalent mass. For instance, in the above examples, the equivalent masses of Chlorine, Sulfur, Nitrogen, and Carbon are respectively 34,45, 32/2 = 16, 14/3 = 4,67, and 12/4 = 3 g/mol. To determine the equivalent (or equivalent mass) of an element, we do not necessarily have to proceed from its compound with Hydrogen. The equivalent (equivalent mass) can be calculated using the composition of a compound of the given element with any other one whose equivalent (equivalent mass) is known. In addition to the concept of equivalent mass, it is sometimes convenient to use the concept of equivalent volume, i.e. the volume occupied in given conditions by one equivalent of the substance being considered. For example, in standard conditions, the equivalent volume of Hydrogen is 11,2, and that of Oxygen is 5,6 litres per mole. The concept of equivalents and equivalent masses also extends to compounds. The equivalent of a compound substance is defined to be an amount of it such that reacts without any residue with one equivalent of Hydrogen or in general with one equivalent of any other substance. The introduction of the concept "equivalent" into chemistry made it possible to formulate a law known as the law of equivalents: The masses (volumes) of substances reacting with one another are proportional to their equivalent masses (volumes): The equivalent of a simple substance and the equivalent of an element (X) in compound (XnYm) are calculated as follows: Ex =Ax /vx (4) The equivalent of a compound (XnYm) is calculated as follows: E XnYm =Mx /nxvx = EX + EY (5) A - atomic mass of element X, M - molecular mass, v - valency of atom X, n - number of atom X in XnYm molecule.
Date: 2015-01-12; view: 1173
|
 A. Einstein
(1879-1955)
A. Einstein
(1879-1955)
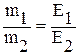 (3)
(3)