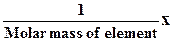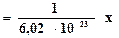CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Molar mass
Three very important concepts - the mole, Avogadro’s number and molar mass - provide the basis for relating masses in grams to numbers of atoms. The first, the mole, is the SI unit for amount of substance. The mole is the amount of a substance that contains the same number of particles as the number of atoms in exactly 12 g of 12C. The abbreviation for the mole is mol. The mole is a counting unit, like one dozen. In writing, a chemist might refer to 1 mol of Carbon, or 2 mol of Iron, or 2,567 mol of calcium. Amount of substance (in mol) is indicated as Greek letter υ.
The number of particles in a mole has been experimentally determinates in a number of ways. The best modern value is 6,022137·1023 particles. This number is of such importance to chemistry that it is named in honor of the Italian scientist Amedeo Avogadro (1776-1856), whose ideas were crucial in the early understanding of chemistry. Avogadro’s number NA- 6,022137·1023 - is the number of particles in exactly one mole of each pure substance. In most cases, Avogadro’s number is rounded to 6,02·1023 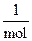 (or mol-1).
(or mol-1).
Molar mass (Mm) is the mass in grams of one mole of pure substance (g/mol). Molar masses of elements contain equal numbers of atoms. One mole of a substance is one molar mass of this substance. An alternative definition of the mole is the amount of a substance that contains an Avogadro’s number of particles of chemical units.
The molar mass is used as the conversion factor in chemical calculations. For a specific substance, a known number of grams can be converted into moles or a known number of moles can be converted into grams. Figure 1 shows the conversions of molar mass, moles, and Avogadro’s number.
In general, mass of any chemical substance (in grams) is connected with amount of this substance by the next ratio:
ν 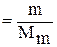 , (1)
, (1)
where ν - amount of any pure substance (mol or kmol);
m - mass of any pure substance (g or kg);
Mm - molar mass (g/mol or kg/kmol).

 | |||
| |||

 | |||
| |||

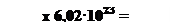
|



|
Figure 1. Relationships between mass, the number of moles, and the number of atoms of an element in the sample
Date: 2015-01-12; view: 1295
| <== previous page | | | next page ==> |
| General notions of atomic-molecular studies | | | EXAMPLE OF SOLUTION |