
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Nomenclature of acids
Nonoxygenous acids
These acids are without oxygen.
For example, HCl – hydrogen is expressed by the prefix hydro- and the name of the main element ending with the suffix –ic because they are only in one form (hydrochloric acid), or the same rule applies as for binary non-metallic compounds (hydrogen chloride). Likewise, HF can either be called hydrofluoric acid or hydrogen fluoride.
Oxoacids
They contain hydrogen, an element forming their name, and oxygen.
This is the case of the older and rather unfortunate way of naming substances, which was briefly mentioned in the chapter about naming binary compounds containing a metal. As you may remember, it uses the endings –ic or –ous, the former referring to a higher oxidation state and the latter to a lower one. Accordingly, H2SO4 is called sulphuric acid (higher oxidation state of sulphur) and H2SO3 sulphurous acid (lower oxidation state). The problem is that you need to know what oxidation states that element really achieves or, more precisely, which oxoacids it really forms. Unfortunately, substances are still named using this system, although the IUPAC (an organisation that sets rules for the nomenclature) is taking steps to change it.
If an element forms more than two oxoacids, the prefixes hypo- and per- are employed.
So e.g. HClO is hypochlorous acid (hypo- refers to a lower oxidation state than the one
marked with the ending –ous)
HClO2 is chlorous acid
HClO3 is chloric acid (-ic referring to a higher oxidation state than –ous)
HClO4 is perchloric acid (per- meaning a higher oxidation state than an acid with
the suffix –ic at the end)
Salts of oxoacids are formed in a very similar way (using partly the older method and partly the stock system). Once you know the name of the acid, simply change its ending: –ous into –ite, and –ic into –ate.
| Oxidation state | Cations and acids | Anions |
| Lowest | hypo- -ous | hypo- -ite |
| -ous | -ite | |
| -ic | -ate | |
| Highest | per- -ic | per- -ate |
Thus, for example, NaClO is called natrium hypochlorite (the name of the element is natrium /sodium and the name of the acid is hypochlorous, so just change its ending from –ous to –ite).
Fe(ClO3)2 is called iron (II) chlorate (mark the oxidation state of iron in the same way as in the stock system of binary compounds with a metal, then take the name of chloric acid (HClO3) and change its ending from –ic to –ate).
22 Name the following compounds:
N2O3
CuCl2
H2CO3
CaCO3
KNO3
Fe(OH)3 ____________
HBrO3
HF
KOH
23 Create formulae of the following compounds:
iron (II) oxide
hydrobromic acid
bromic acid
sulphuric acid
diphosphorus pentaoxide
carbon (mono)oxide
sulphur tetraiodide
lead (II) iodide
24 Match the chemical formulae with the correct name and the definition:
1) MgO citric acid a) it is a white crystalline solid; it is a major
chemical in the world and one of the most
damaging salts in structure conservation; the
hydrate is known as Glauber´s Salt
2) HCL acetylene b) in the lower atmosphere is an air pollutant
with harmful effects on the respiratory
systems of animals, it can burn sensitive
plants; it is a pale blue gas soluble in water
3) CaCl2 sodium chloride c) it is used for relief of heartburn and sore
stomach ; to improve symptoms of
indigestion
4) CO2 sodium sulphate d) it is a colourless gas which forms white
fumes ; skin contact can cause redness,
pain, severe skin burns
5) C2H2 trioxygen e) it is a weak organic acid and a natural
preservative; it is also used to add an
acidic, or sour taste to food and soft
drinks; it exists in a variety of fruits
6) NaCl magnesium oxide f) it is a colourless gas widely used as a
fuel; it is mainly manufactured by the
particial combustion of methane; it has
explosive character and ability to poison
7) Na2SO4 ethanol g) it is solid at room temperature; it can be
produced directly from limestone; as an
ingredient it is listed as a permitted food
additive in EU as E509
8) O3 hydrogen chloride h) it is a gas at standard temperature and
pressure; it exists in Earth´s atmosphere
in this state; it is known as a part of
photosynthesis
9) C2H5OH calcium chloride i) it is essential for animal life in small
quantities; it can be harmful to animals
and plants in excess; it is used for food
preservation
10) C6H8O7 carbon dioxide j) it is also called pure alcohol; it is
a flammable, colourless liquid, known as
an essential solvent; it is used in
medicine, food industry, etc.
25 Study mathematical terms, units, symbols.
Calculations
9 + 2 = nine plus two equals / is equal to
20 – 7 = twenty minus seven equals / is equal to
4 x 36 = four multiplied by thirty-six equals / is equal to
10 : 5 = ten divided by five equals / is equal to
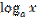 = log base a of x equals / is equal to
= log base a of x equals / is equal to
Powers and roots
x2 x squared
x3 x cubed
x4 x to the power (of) four / x to the four / x to the fourth (power)
x-5 x to the power (of) minus five / x to the minus five / x to the fifth (power)
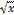 the square root of x
the square root of x
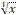 the cube root of x
the cube root of x
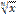 the nth root of x
the nth root of x
Numbers
100 a / one hundred
101 a hundred and one and is often left out in AmE
4,938 four thousand nine hundred and thirty-eight
5,405 five thousand four hundred and five
Ordinals
1st the first
2nd the second
3rd the third
24th the twenty-fourth
Decimals
0.25 nought / zero point two five After the point, say numbers separately.
Fractions
 a / one half
a / one half
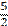 five halves
five halves
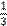 a / one third
a / one third
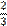 two thirds
two thirds
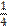 a / one quarter
a / one quarter
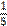 a / one fifth
a / one fifth
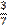 three sevenths
three sevenths
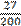 twenty-seven over two hundred Complex fractions are usually said with over.
twenty-seven over two hundred Complex fractions are usually said with over.
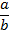 a over b
a over b
Percentages
27 % twenty-seven per cent
Temperature
95° C ninety-five degrees Celsius/Centigrade
13° F thirteen degrees Fahrenheit
Units
mol mole
mol/dm3 a mole per cubic decimetre / AmE decimeter
ml millilitre / AmE milliliter
Symbols
( ) round brackets
[ ] square brackets
{} curly brackets
(A+B) open brackets, A plus B, close brackets / A+B in brackets
A < B A is less than B
A > B A is greater than B
A ≠ B A is not equal to B
A ≈ B A is approximately equal to B
→ give(s), lead(s) to, yield(s)
↔ forms and is formed from
A capital A
a small a
p1V1 = p2V2 small p subscript one capital V subscript one equals small p subscript two capital
V subscript two

Chemical formulae can also be read with the help of spelling:
e.g. 2 Na2S could be read as two molecules of [en ei tu: es]
26 Read the following expressions.

 25,326 58th 1.012 15,123,014 76 100°C 5,093 15.012
25,326 58th 1.012 15,123,014 76 100°C 5,093 15.012
 6 H2O 3 H2SO3
6 H2O 3 H2SO3  8% 2 KClO3 → 2 KCl + 3 O2 14-2
8% 2 KClO3 → 2 KCl + 3 O2 14-2 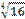
9 mol/dm3 5 Cu (NO3)2
27 Read the article and do the following exercises:
a) Make collocations used in the article.
1. cell a patterns
2. marine b technologies
3. UV c field
4. solar d nanostructures
5. new e walls
6. silica f algae
7. to diffract g radiation
8. magnetic h light
9. multilayer i crystals
10. photonic j energy
1 __ 2 __ 3 __ 4 __ 5 __ 6 __ 7 __ 8 __ 9 __ 10 __
b) Fill in the missing prepositions. You can consult the article.
1. ___ a scale of around 100 nm
2. rely ___ nanoscale effects
3. seen ___ nature
4. studied ___ researchers
5. 100-200 nm ___ size
6. ___ the University of Utah ___ 2008
7. ___ any angle
8. work ____ the lab
9. made ____ crystals
10. coated ____ a layer
c) Scan through the article and find synonyms of the following expressions:
1. effectively
2. making possible
3. cleared away
4. to have or own something
5. to manufacture
6. to start, to launch
7. actually
8. to be supposed
9. to look at carefully in order to find out something
d) Form antonyms of the following words from the article:
1. natural
2. better
3. dim
4. strong
5. useful
6. exactly
7. visible
8. commonly
9. inner
e) In the article find the words corresponding to the following definitions:
1. very simple, usually very small plants that live in or near water; they have no roots, stems
or leaves
2. the outer part of an object
3. a set of animals or plants in which the members have similar characteristics to each other
4. having an empty space inside
5. to increase the strength of something
f) Form other parts of speech from the words used in the article.
1. are assumed noun:
2. are discovering noun:
3. occurs noun:
4. developing noun:
5. diffract noun:
6. a damage verb:
7. applications verb:
8. reflectance verb:
9. a cell adjective:
10. nature adjective:
g) Look at the phrases below. With your partner, try to recall exactly how these were used
in the article.
1. drug delivery tools
2. the hydrophobicity of surfaces
3. a self-cleaning plant
4. nano-sized photonic crystals
5. the wavelength of UV radiation
h) Summarize the article.
28 Unscramble the nouns below and explain their meaning in English.
1. cipipretant _______________ 3. rivadetive _________________
2. antcatre __________________ 4. mopocnent _________________
29 Explain the jokes about chemists and chemistry.
question: Why are chemists good at solving problems?
answer: They have all the solutions.
question: If H-two-O is the formula for water, what is the formula for ice?
answer: H-two-O cubed.
teacher: “What is the formula for water?”
student: “H,I,J,K,L,M,N,O”
teacher: “That´s not what I taught you! ”
student: “But you said the formula for water was H – to – O. ”
A chemist walks into a pharmacy and asks the pharmacist:
“Do you have any acetylsalicylic acid?”
“Do you mean aspirin?” asked the pharmacist.
“That´s it, I can never remember the word.” answered the chemist.
teacher: “What does HNO3 signify?”
student: “Well, …ah, … I´ve got it right on the tip of my tongue.”
teacher: “Well, you had better spit it out.”
Why did the white bear dissolve in water?
Because it was polar.
GRAMMAR LINKS
30 Study two sections of the Grammar File - Passive voice and Relative clauses.
UNIT 3
Date: 2015-01-12; view: 3570
| <== previous page | | | next page ==> |
| Study the nomenclature of inorganic chemistry. | | | LABORATORY |