
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
COMPLEX (COORDINATE) COMPOUNDS
By complex (coordinate) compounds are meant definite chemical compounds formed by a combination of individual components without formation of new pairs of electrons. Examples of complex compounds are Na3[Co(NO2)6], [Cu(NH3)4]SO4, K4[Fe(CN)6]. In a molecule of a complex compound, one of the atoms, generally positively charged, occupies the central site (central ion or complexing agent). Oppositely charged ions or neutral molecules called ligands are coordinated around the central ion. The complexing agent and the ligands form inner sphere of a complex compound. It is characterized by coordinate bonds which are formed while overlapping of empty p- and d-orbitals of a central ion and orbitals containing lone electron pairs of ligands. The ions in the outer sphere are mainly bonded to the complex ions by forces of electrostatic interaction (ionic bonds). The total number of coordinate bonds formed by the complexing agent with ligands is known as coordination number of the central ion. In accordance with the number of coordinate bonds formed by a ligand with the central ion, the ligand may be a monodentate, bidentate, or polydentate.
Majority of complex compounds are electrolytes. In solutions they dissociate and form both simple and complex ions (outer and inner spheres). This type of dissociation is an irreversible process (complex compounds are strong electrolytes). [Cu(NH3)4]SO4
The inner sphere of a complex compound dissociates reversibly and stepwise (complex ions are weak electrolytes):
[Cu(NH3)4]2+ [Cu(NH3)3]2+ [Cu(NH3)2]2+ [Cu(NH3)]2+ Each of the above processes can be characterized by an equilibrium constant (stepwise instability constants of a complex ion). The equilibrium constant of the overall process is called an overall instability constant of a complex ion. The less the value of the overall instability constant is, the more stable is the complex ion. The shifting of dissociation equilibrium in systems containing complex ions follows the same rules as in solutions of simple (non-complex) electrolytes, namely: equilibrium shifts towards the direction of the most complete binding of the complexing agent or ligand so that the concentrations of these particles remaining unbound in the solution take on the minimum possible values in these conditions. The equilibrium also shifts towards the formation of a more stable complex ion. According to the character of dissociation of complex compounds, cationic, anionic and neutral complexes are distinguished. In cationic complexes ligands are usually neutral molecules so that the inner sphere is charged positively: [Cr(H2O)6]Cl3, [Co(NH3)6]Cl3. In anionic complexes ligands are usually negatively charged: K2[HgI4], Na[Sb(OH)6]. Neutral complexes have both anions and neutral molecules as ligands: [Pt(NH3)2Cl2]. They have no outer sphere and donít dissociate in aqueous solutions.
EXPERIMENTAL PART
1. Formation of complex compounds
Put 4-5 drops of silver nitrate into a test tube. Add ammonium hydroxide until the precipitate is formed. Add excess of NH4OH and observe the formation of a complex compound. Repeat the procedure with CuSO4, NiCl2, Zn(NO3)2. Write down the reactions. Find the values of overall instability constants and compare stabilities of the above complex compounds.
Notice: (a) instead of Cu(OH)2, (CuOH)2SO4 precipitate is formed; (b) coordination number of silver ion is 2; for Cu2+ and Zn2+ it is 4; for Ni2+ - 6.
2. Destruction of complex compounds
Take solutions of chloride diammine silver (I) and sulphate tetraammine copper (II), obtained from the previous experiment, add dropwise diluted (1:1) nitric acid until the complex compounds are destroyed.
3. Formation and properties of Cd2+ and Hg2+ complexes
Take two test tubes filled with solutions of Cd2+ and Hg2+ salts respectively. Add a saturated solution of Na2SO3. Observe the formation of sulfide precipitates and their further dissolution with the formation of complex compounds Na2[M(SO3)2], where M= Cd2+ Add some drops of NaOH to the solutions of complex compounds. Do metal hydroxides precipitate? Why? 4. Dissociation of complex compounds
a). Fill 3 test tubes with 3-5 drops of a double salt NH4Fe(SO4)2. Add 3-4 drops of NaOH into the first test tube and heat the solution. Smell the vapors. Write down your observations. Add 2-3 drops of BaCl2 to the second test tube and 2-3 drops of KSCN to the third one. Make your conclusion about the dissociation of double salts in aqueous solutions. Write down the reactions of dissociation of a double salt and reactions of ionic exchange.
b). Fill 2 test tubes with 3-5 drops of K3[Fe(CN)6]. Add 2-3 drops of Na3[Co(NO2)6] and 2-3 drops of KSCN to the second one. Write down your observations and the reactions of dissociation of a complex compound and ionic exchange. (Notice: in presence of K+ ions, the yellow precipitate of K2Na[Co(NO2)6] is formed.)
QUESTIONS AND PROBLEMS 1. For the following complex compounds indicate: (a) inner and outer spheres; (b) central ion, its charge and coordinating number; (c) ligands ; (d) write the reaction of dissociation of complex compounds and complex ions; (e) express overall instability constants; (f) name the following complex compounds. [Cd(NH3)4]Cl2 ; K2[Cd(CN)4] 2. Which of the above mentioned complexes is more stable? 3. Calculate the concentration of the Ag+ ions in a 0.1 M solution containing an excess of 1 mole × l-1 of NH3. 4. In which case will a reaction occur between solutions of the electrolytes indicated below (exchange of ligands)? Write the equations of these reactions in molecular and net ionic forms and calculate their equilibrium constants: (a) K2[HgI4] + KCN = (b) K[Ag(CN)2] + NH3 = Experiment 6 S-ELEMENTS (ALKALINE and ALKALINE EARTH METALS)
Alkaline metals Li, Na, K, Rb, Cs, Fr are situated in the IA group of the periodic table. The outer spheres of these atoms consist of only one electron. So atoms of alkaline metals have a tendency to lose their valence electrons to be transformed into positively charged ions. Their oxidation number is +1. The strength of the attraction of the outer electron to the atom can be valued with the help of the ionization potential which determines the energy of removal of one electron from a neutral atom. In the IA group from Li to Fr the value of ionization potential decreases, the chemical activity of metals increases. Alkaline metals are strong reducing agents. In air alkaline metals are easily oxidized, that is why they are stored under oil. Alkaline metals are more active than hydrogen (they have negative values of redox-potentials) so they can replace hydrogen both from acids and water: 2M + HCl = 2MCl + H2 2M + 2H2O = 2MOH + H2 In aqueous solutions metal hydroxides behave as strong electrolytes and are fully dissociated: MOH Almost all salts of alkaline metals are water soluble. Solutions of salts containing anions of weak acids undergo hydrolysis; they are basic. Pure alkaline metals are produced by electrolysis of their melted salts.
Biological significance of alkaline metals Lithium is found in the liver and lungs of animals. Large concentrations of lithium are dangerous for humans . Particles of dust and smoke containing lithium provoke malignant tumors. Sodium as NaCl is necessary for the balance of salt exchange in organisms, sodium bicarbonate is used to lower the acidity of gastric juice. In living organisms potassium is situated in liver, spleen, it regulates the function of muscle cells and nervous systems. Rubidium is found in the leaves of plants (beetroot, sugar-cane, tabacoo, tea, coffee, cocoa). In animal organisms it is localized in muscles performing large load - heart muscle and pectoral muscles of birds. Cesium is found in mineral water, plants and living organisms. Compounds of rubidium and cesium are necessary for the growth of plants.
Alkaline earth metals Ca, Sr, Ba, Ra are situated in the IIA group of the periodic table. Atoms of these elements have two valence electrons. While losing them, atoms of alkaline earth elements transfer into positively charged ions and gain the oxidation number +2. The presence of non-filled sublevels (d- and f-) makes Ca, Sr, Ba and Ra more chemically active and having physical properties rather different than those of Be and Mg. Metals of IIA group are less active than alkaline metals. The growth of the atomic radius, lowering of the ionization potential and of the electronegativity define the growth of their chemical activity with increase of charges of the nuclei. In the air alkaline earth metals easily form oxides. They replace hydrogen both from water and from acids: M +2HCl = MCl2 + H2 M + 2H2O = M(OH)2 + H2 The basic character of oxides and hydroxides increases with the increase of atomic radii from calcium to radium. Alkaline earth metals form different sparingly soluble salts: carbonates, phosphates, chromates, sulfates. The solubility of sulfates lowers from calcium to radium. Natural water containing soluble salts of calcium and magnum is called hard water. The presence of hydrocarbonates of calcium and magnum stipulates the temporary hardness of water. Chlorides and sulfates of these elements cause the constant hardness. The sum of temporary and constant hardness gives the overall hardness of water.
Biological and agricultural properties of elements of IIA group Beryllium and its compounds are toxic. The beryllium-poisoning may cause the death. Magnum compounds can be found in algae, fungi, ferns, in the tissues of animals. Magnum is a complexing ion in chlorophyll. Calcium is a constituent of the bones of vertebrates, predominantly as ortophosphate Ca3(PO4)2. Egg-shells, tests of sea animals, shells mainly consist of calcium carbonate. Organic salts of calcium play a significant role in metabolism of plants. The deficiency in calcium leads to stopping of their growth, development of rhizomes, the leaves cover by brown spots and die off. The animals suffer from rachitis, the heart activity decreases, the blood coagulability becomes worse. Calcium ions enter human organisms with milk and meat meals while magnum ions - with vegetable meal. Strontium compounds in human organisms are mainly concentrated in bones, the excess (more than 10-3 %) leads to their fragile. There are approximately 100 times less barium compounds in human bodies than those of strontium. In very small quantities barium compounds stimulate activity of marrow. In large quantities they are very toxic and provoke weakness, gastric-intestinal diseases, brain disorders. Barium chloride and carbonate are used in the agriculture as chemical weed-killers and pest-killers.
EXPERIMENTAL PART
1. Interaction of sodium with air and water (WARNING: put on protective glasses!)
a) Take a piece of sodium from a vessel with oil (use pincers), put it on a filter paper and cut it with a knife. What happens to the shining surface of sodium after some time? b) Put a small piece of sodium into a porcelain dish filled with water. Add 1-2 drops of phenolphtalein as indicator.
2. Hydrolysis of salts of alkaline metals
Take some crystals of sodium acetate into a test tube and add distilled water until the salt dissolves. Measure pH of the solution with the help of the universal indicator. Repeat the experiment with sodium and potassium carbonates.
3. Decomposition of nitrates of alkaline metals
Take about 1 g of sodium nitrate into a test tube and fix it in a stand. Heat the test tube in a flame and examine the evaluation of oxygen.
4. Interaction of calcium with water
Take some distilled water into a porcelain dish and add some drops of phenolphtalein. Put a small piece of calcium into the cup (use pincers). Compare the rate of the evaluation of hydrogen with the one from experiment 1b.
5. Formation of carbonates
Fill 3 test tubes with 1 ml of solutions of calcium, strontium and barium salts respectively and add 1 ml of sodium carbonate solution into each test tube. Try to dissolve the formed precipitates in hydrochloric acid.
6. Formation of calcium and barium oxalates
Fill 2 test tubes with 1 ml of the solutions of calcium and barium salts respectively and add 1 ml of ammonium oxalate (NH4)2C2O4. Divide each precipitate into two parts and try to dissolve them in hydrochloric and acetic acids.
7. Formation of gypsum
Put 1-2 ml of a saturated solution of calcium chloride in a test tube and add 1-2 ml of 50% solution of sulfuric acid. Observe the formation of the crystals of gypsum.
QUESTIONS AND PROBLEMS 1. How does the value of the ionization potential changes with the change of the position of an element in the periodic table? 2. Which are the rules of storage and handling of alkaline and alkaline earth metals? 3. How are compounds of alkaline and alkaline earth metals are used in the medical practice? 4. Calculate pH of 0.01 M solution of sodium acetate. 5. Calculate the equilibrium constants of the reactions and explain why the precipitate of barium chromate can be dissolved in hydrochloric acid and canít be dissolved in acetic acid.
Date: 2015-01-12; view: 1080
|

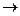 [Cu(NH3)4]2+ + SO42
[Cu(NH3)4]2+ + SO42 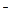
 [Cu(NH3)3]2+ + NH3
[Cu(NH3)3]2+ + NH3