CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Oxides and oxyanionsThe principal sulfur oxides are obtained by burning sulfur: S + O2 → SO2 2 SO2 + O2 → 2 SO3 Other oxides are known, e.g. sulfur monoxide and disulfur mono- and dioxides, but they are unstable. The sulfur oxides form numerous oxyanions with the formula SOn2–. Sulfur dioxide and sulfites (SO2−3) are related to the unstable sulfurous acid (H2SO3). Sulfur trioxide and sulfates (SO2−4) are related to sulfuric acid. Sulfuric acid and SO3 combine to give oleum, a solution of pyrosulfuric acid(H2S2O7) in sulfuric acid.
Picture 7. Peroxydisulfuric acid Peroxides convert sulfur into unstable such as S8O, a sulfoxide. Peroxymonosulfuric acid (H2SO5) and peroxydisulfuric acids (H2S2O8), made from the action of SO3 on concentrated H2O2, and H2SO4 on concentrated H2O2 respectively.
Picture 8. The sulfate anion, SO2−4 Thiosulfate salts (S2O2-3), sometimes referred as "hyposulfites", used in photographic fixing (HYPO) and as reducing agents, feature sulfur in two oxidation states. Sodium dithionite, (S2O2-4), contains the more highly reducing dithionite anion. Sodium dithionate (Na2S2O6) is the first member of the polythionic acids (H2SnO6), where n can range from 3 to many. Halides and oxyhalides The two main sulfur fluorides are sulfur hexafluoride, a dense gas used as nonreactive and nontoxic propellant, and sulfur tetrafluoride, a rarely used organic reagent that is highly toxic. Their chlorinated analogs are sulfur dichloride and sulfur monochloride. Sulfuryl chloride and chlorosulfuric acidare derivatives of sulfuric acid; thionyl chloride (SOCl2) is a common reagent in organic synthesis. Pnictides An important S–N compound is the cage tetrasulfur tetranitride (S4N4). Heating this compound gives polymeric sulfur nitride ((SN)x), which has metallic properties even though it does not contain any metal atoms. Thiocyanates contain the SCN− group. Oxidation of thiocyanate gives thiocyanogen, (SCN)2 with the connectivity NCS-SCN. Phosphorus sulfides are numerous, the most important commercially being the cages P4S10 and P4S3. Metal sulfides The principal ores of copper, zinc, nickel, cobalt, molybdenum, and other metals are sulfides. These materials tend to be dark-colored semiconductorsthat are not readily attacked by water or even many acids. They are formed, both geochemically and in the laboratory, by the reaction of hydrogen sulfide with metal salts. The mineral galena (PbS) was the first demonstrated semiconductor and found a use as a signal rectifier in the cat's whiskers of early crystal radios. The iron sulfide called pyrite, the so-called "fool's gold," has the formula FeS2.[30] The upgrading of these ores, usually by roasting, is costly and environmentally hazardous. Sulfur corrodes many metals via the process called tarnishing.
Organic compounds
Allicin, the active ingredient in garlic
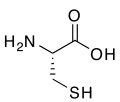
R-cysteine, an amino acidcontaining a thiol group
Methionine, an amino acidcontaining a thioether
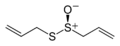
Diphenyl disulfide, a representative disulfide
Perfluorooctanesulfonic acid, a controversial surfactant
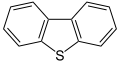
Dibenzothiophene, a component of crude oil
Penicillin Some of the main classes of sulfur-containing organic compounds include the following:
Compounds with carbon–sulfur bonds are uncommon with the notable exception of carbon disulfide, a volatile colorless liquid that is structurally similar to carbon dioxide. It is used as a reagent to make the polymer rayon and many organosulfur compounds. Unlike carbon monoxide, carbon monosulfide is only stable as a dilute gas, as in the interstellar medium. Organosulfur compounds are responsible for the some of the unpleasant odors of decaying organic matter. They are used in the odoration of natural gas and cause the odor of garlic and skunk spray. Not all organic sulfur compounds smell unpleasant at all concentrations: the sulfur-containing monoterpenoid grapefruit mercaptan in small concentrations is responsible for the characteristic scent of grapefruit, but has a generic thiol odor at larger concentrations. Sulfur mustard, a potent vesicant, was used in World War I as a disabling agent. Sulfur-sulfur bonds are a structural component to stiffen rubber, in a way similar to the biological role of disulfide bridges to rigidify proteins (see biological below). In the most common type of industrial "curing" or hardening and strengthening of natural rubber, elemental sulfur is heated with the rubber to the point that chemical reactions form disulfide bridges between isoprene units of the polymer. This process, patented in 1843, allowed rubber to become a major industrial product, especially automobile tires. Because of the heat and sulfur, the process was namedvulcanization, after the Roman god of the forge and volcanism. History Antiquity
Picture 9. Pharmeceutical container for sulfur from the first half of the 20th century. Being abundantly available in native form, sulfur (Latin sulphur) was known in ancient times and is referred to in the Torah (Genesis). English translations of the Bible commonly referred to burning sulfur as "brimstone", giving rise to the name of 'fire-and-brimstone' sermons, in which listeners are reminded of the fate of eternal damnation that await the unbelieving and unrepentant. It is from this part of the Bible that Hell is implied to "smell of sulfur" (likely due to its association with volcanic activity). According to the Ebers Papyrus, a sulfur ointment was used in ancient Egypt to treat granular eyelids. Sulfur was used for fumigation in preclassical Greece; this is mentioned in the Odyssey. Pliny the Elder discusses sulfur in book 35 of his Natural History, saying that its best-known source is the island of Melos. He mentions its use for fumigation, medicine, and bleaching cloth. A natural form of sulfur known as shiliuhuang was known in China since the 6th century BC and found in Hanzhong. By the 3rd century, the Chinese discovered that sulfur could be extracted from pyrite. Chinese Daoists were interested in sulfur's flammability and its reactivity with certain metals, yet its earliest practical uses were found in traditional Chinese medicine. A Song Dynasty military treatise of 1044 AD described different formulas for Chinese black powder, which is a mixture of potassium nitrate (KNO3), charcoal, and sulfur. Indian alchemists, practitioners of "the science of mercury" wrote extensively about the use of sulfur in alchemical operations with mercury, from the eighth century AD onwards. In the rasaśāstra tradition, sulphur is called "the smelly," Early European alchemists gave sulfur its own alchemical symbol, a triangle at the top of a cross. In traditional skin treatment before the modern era of scientific medicine, elemental sulfur was used, mainly in creams, to alleviate conditions such as scabies, ringworm, psoriasis, eczema, and acne. The mechanism of action is unknown—though elemental sulfur does oxidize slowly to sulfurous acid, which in turn (through the action of sulfite) acts as a mild reducing and antibacterial agent. Modern times
Picture 10. Sicilian kiln used to obtain sulfur from volcanic rock. In 1777, Antoine Lavoisier helped convince the scientific community that sulfur was an element, not a compound. With the sulfur from Sicily being principally controlled by the French market, a debate ensued about the amount of sulfur France and Britain got. This led to a bloodless confrontation between the two sides in 1840. In 1867, sulfur was discovered in underground deposits in Louisiana and Texas. The highly successful Frasch process was developed to extract this resource. In the late 18th century, furniture makers used molten sulfur to produce decorative inlays in their craft. Because of the sulfur dioxide produced during the process of melting sulfur, the craft of sulfur inlays was soon abandoned. Molten sulfur is sometimes still used for setting steel bolts into drilled concrete holes where high shock resistance is desired for floor-mounted equipment attachment points. Pure powdered sulfur was used as a medicinal tonic and laxative.[16] With the advent of the contact process, the majority of sulfur today is used to make sulfuric acid for a wide range of uses, particularly fertilizer.
Date: 2016-04-22; view: 1645
|