CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Commercial chemistryColor
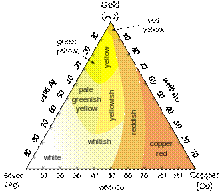
Different colors of Ag-Au-Cu alloys Whereas most other pure metals are gray or silvery white, gold is yellow. This color is determined by the density of loosely bound (valence) electrons; those electrons oscillate as a collective "plasma" medium described in terms of a quasiparticle called plasmon. The frequency of these oscillations lies in the ultraviolet range for most metals, but it falls into the visible range for gold due to subtle relativistic effects that affect the orbitals around gold atoms.[24][25] Similar effects impart a golden hue to metallic caesium (see relativistic quantum chemistry). Common colored gold alloys such as rose gold can be created by the addition of various amounts of copper and silver, as indicated in the triangular diagram to the left. Alloys containing palladium or nickel are also important in commercial jewelry as these produce white gold alloys. Less commonly, addition of manganese, aluminium, iron, indium and other elements can produce more unusual colors of gold for various applications.[22] Isotopes Main article: Isotopes of gold Gold has only one stable isotope, 197Au, which is also its only naturally occurring isotope. Thirty-six radioisotopes have been synthesized ranging in atomic mass from 169 to 205. The most stable of these is 195Au with a half-life of 186.1 days. The least stable is 171Au, which decays by proton emission with a half-life of 30 µs. Most of gold's radioisotopes with atomic masses below 197 decay by some combination of proton emission, α decay, and β+ decay. The exceptions are 195Au, which decays by electron capture, and 196Au, which decays most often by electron capture (93%) with a minor β- decay path (7%).[26] All of gold's radioisotopes with atomic masses above 197 decay by β- decay.[27] At least 32 nuclear isomers have also been characterized, ranging in atomic mass from 170 to 200. Within that range, only 178Au, 180Au, 181Au, 182Au, and 188Au do not have isomers. Gold's most stable isomer is 198m2Au with a half-life of 2.27 days. Gold's least stable isomer is 177 m2Au with a half-life of only 7 ns. 184 m1Au has three decay paths: β+ decay, isomeric transition, and alpha decay. No other isomer or isotope of gold has three decay paths.[27] Applications Monetary exchange Gold has been widely used throughout the world as a vehicle for monetary exchange, either by issuance and recognition of gold coins or other bare metal quantities, or through gold-convertible paper instruments by establishing gold standards in which the total value of issued money is represented in a store of gold reserves. The first gold coins of the Grecian age were struck in Lydia around 700 BC.[28] The talent coin of gold in use during the periods of Grecian history both before and during the time of the life of Homer weighed between 8.42 and 8.75 grams.[29] From an earlier preference in using silver, European economies re-established the minting of gold as coinage during the thirteenth and fourteenth centuries.[30] However, production has not grown in relation to the world's economies. Today, gold mining output is declining.[31] With the sharp growth of economies in the 20th century, and increasing foreign exchange, the world's gold reserves and their trading market have become a small fraction of all markets and fixed exchange rates of currencies to gold were no longer sustained. At the beginning of World War I the warring nations moved to a fractional gold standard, inflating their currencies to finance the war effort. After World War II gold was replaced by a system of convertible currency following the Bretton Woods system. Gold standards and the direct convertibility of currencies to gold have been abandoned by world governments, being replaced by fiat currency in their stead. Switzerland was the last country to tie its currency to gold; it backed 40% of its value until the Swiss joined the International Monetary Fund in 1999.[32] Pure gold is too soft for day-to-day monetary use and is typically hardened by alloying with copper, silver or other base metals. The gold content of alloys is measured in carats (k). Pure gold is designated as 24k. English gold coins intended for circulation from 1526 into the 1930s were typically a standard 22k alloy called crown gold,[33] for hardness (American gold coins for circulation after 1837 contained the slightly lower amount of 0.900 fine gold, or 21.6 kt).[34] Although the price of some platinum group metals can be much higher, gold has long been considered the most desirable of precious metals, and its value has been used as the standard for many currencies (known as the gold standard) in history. Gold has been used as a symbol for purity, value, royalty, and particularly roles that combine these properties. Gold as a sign of wealth and prestige was ridiculed by Thomas More in his treatise Utopia. On that imaginary island, gold is so abundant that it is used to make chains for slaves, tableware and lavatory-seats. When ambassadors from other countries arrive, dressed in ostentatious gold jewels and badges, the Utopians mistake them for menial servants, paying homage instead to the most modestly dressed of their party. Investment Main article: Gold as an investment
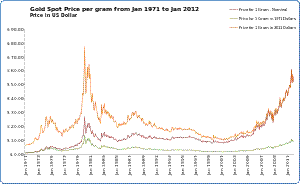
Gold price per gram between Jan 1971 and Jan 2012. The graph shows nominal price in US dollars, the price in 1971 and 2011 US dollars Many holders of gold store it in form of bullion coins or bars as a hedge against inflation or other economic disruptions. However, the economist Martin Feldstein does not believe gold serves as a hedge against inflation or currency depreciation.[35] The ISO 4217 currency code of gold is XAU. Modern bullion coins for investment or collector purposes do not require good mechanical wear properties; they are typically fine gold at 24k, although the American Gold Eagle and the British gold sovereign continue to be minted in 22k metal in historical tradition, and the South African Krugerand, first released in 1967, is also 22k.[36] The special issueCanadian Gold Maple Leaf coin contains the highest purity gold of any bullion coin, at 99.999% or 0.99999, while thepopular issue Canadian Gold Maple Leaf coin has a purity of 99.99%. Several other 99.99% pure gold coins are available. In 2006, the United States Mint began production of the American Buffalo gold bullion coin with a purity of 99.99%. The Australian Gold Kangaroos were first coined in 1986 as the Australian Gold Nugget but changed the reverse design in 1989. Other popular modern coins include the Austrian Vienna Philharmonic bullion coin and the Chinese Gold Panda. Jewelry Main article: Jewelry
Moche gold necklace depicting feline heads. Larco Museum Collection. Lima-Peru Because of the softness of pure (24k) gold, it is usually alloyed with base metals for use in jewelry, altering its hardness and ductility, melting point, color and other properties. Alloys with lower caratage, typically 22k, 18k, 14k or 10k, contain higher percentages of copper, or other base metals or silver or palladium in the alloy. Copper is the most commonly used base metal, yielding a redder color.[37] Eighteen-carat gold containing 25% copper is found in antique and Russian jewelry and has a distinct, though not dominant, copper cast, creating rose gold. Fourteen-carat gold-copper alloy is nearly identical in color to certain bronze alloys, and both may be used to produce police and other badges. Blue gold can be made by alloying with iron and purple gold can be made by alloying with aluminium, although rarely done except in specialized jewelry. Blue gold is more brittle and therefore more difficult to work with when making jewelry.[37] Fourteen and eighteen carat gold alloys with silver alone appear greenish-yellow and are referred to as green gold. White gold alloys can be made with palladium or nickel. White 18-carat gold containing 17.3% nickel, 5.5% zinc and 2.2% copper is silvery in appearance. Nickel is toxic, however, and its release from nickel white gold is controlled by legislation in Europe.[37] Alternative white gold alloys are available based on palladium, silver and other white metals,[37] but the palladium alloys are more expensive than those using nickel. High-carat white gold alloys are far more resistant to corrosion than are either pure silver or sterling silver. The Japanese craft of Mokume-gane exploits the color contrasts between laminated colored gold alloys to produce decorative wood-grain effects. Medicine Conceived of as perhaps the most anciently administered medicine (apparently according to one source by shamanic practitioners)[38] and known of by Dioscorides,[39][40]apparent paradoxes of the actual toxicology of the substance nevertheless suggests the possibility still of serious gaps in understanding of action on physiology.[41] In medieval times, gold was often seen as beneficial for the health, in the belief that something so rare and beautiful could not be anything but healthy. Even some modernesotericists and forms of alternative medicine assign metallic gold a healing power.[42] Some gold salts do have anti-inflammatory properties and are used as pharmaceuticals in the treatment of arthritis and other similar conditions. Gold based injections have been explored as a means to help to reduce the pain and swelling of rheumatoid arthritis andtuberculosis.[43] However, only salts and radioisotopes of gold are of pharmacological value, as elemental (metallic) gold is inert to all chemicals it encounters inside the body. Gold alloys are used in restorative dentistry, especially in tooth restorations, such as crowns and permanent bridges. The gold alloys' slight malleability facilitates the creation of a superior molar mating surface with other teeth and produces results that are generally more satisfactory than those produced by the creation of porcelain crowns. The use of gold crowns in more prominent teeth such as incisors is favored in some cultures and discouraged in others. Colloidal gold preparations (suspensions of gold nanoparticles) in water are intensely red-colored, and can be made with tightly controlled particle sizes up to a few tens of nanometers across by reduction of gold chloride with citrate or ascorbate ions. Colloidal gold is used in research applications in medicine, biology and materials science. The technique of immunogold labeling exploits the ability of the gold particles to adsorb protein molecules onto their surfaces. Colloidal gold particles coated with specific antibodies can be used as probes for the presence and position of antigens on the surfaces of cells.[44] In ultrathin sections of tissues viewed by electron microscopy, the immunogold labels appear as extremely dense round spots at the position of the antigen.[45] Gold, or alloys of gold and palladium, are applied as conductive coating to biological specimens and other non-conducting materials such as plastics and glass to be viewed in ascanning electron microscope. The coating, which is usually applied by sputtering with an argon plasma, has a triple role in this application. Gold's very high electrical conductivity drains electrical charge to earth, and its very high density provides stopping power for electrons in the electron beam, helping to limit the depth to which the electron beam penetrates the specimen. This improves definition of the position and topography of the specimen surface and increases the spatial resolution of the image. Gold also produces a high output of secondary electrons when irradiated by an electron beam, and these low-energy electrons are the most commonly used signal source used in the scanning electron microscope.[46] Nuclear medicine : The isotope gold-198 (half-life 2.7 days) is used, in nuclear medicine, in some cancer treatments and for treating other diseases.[47][48] Food and drink · Gold can be used in food and has the E number 175.[49] · Gold leaf, flake or dust is used on and in some gourmet foods, notably sweets and drinks as decorative ingredient.[50] Gold flake was used by the nobility in medieval Europe as a decoration in food and drinks, in the form of leaf, flakes or dust, either to demonstrate the host's wealth or in the belief that something that valuable and rare must be beneficial for one's health. · Danziger Goldwasser (German: Gold water of Danzig) or Goldwasser (English: Goldwater) is a traditional German herbal liqueur[51] produced in what is today Gdańsk, Poland, and Schwabach, Germany, and contains flakes of gold leaf. There are also some expensive (~$1000) cocktails which contain flakes of gold leaf.[52] However, since metallic gold is inert to all body chemistry, it has no taste, it provides no nutrition, and it leaves the body unaltered.[53] Industry
The 220kg gold brick displayed inJinguashi Gold Museum, Taiwan, Republic of China.
The world's largest gold barhas a mass of 250 kg. Toi museum, Japan.
A gold nugget of 5 mm in diameter (bottom) can be expanded through hammering into a gold foil of about 0.5 square meters. Toi museum, Japan. · Gold solder is used for joining the components of gold jewelry by high-temperature hard soldering or brazing. If the work is to be ofhallmarking quality, gold solder must match the carat weight of the work, and alloy formulas are manufactured in most industry-standard carat weights to color match yellow and white gold. Gold solder is usually made in at least three melting-point ranges referred to as Easy, Medium and Hard. By using the hard, high-melting point solder first, followed by solders with progressively lower melting points, goldsmiths can assemble complex items with several separate soldered joints. · Gold can be made into thread and used in embroidery. · Gold produces a deep, intense red color when used as a coloring agent in cranberry glass. · In photography, gold toners are used to shift the color of silver bromide black-and-white prints towards brown or blue tones, or to increase their stability. Used on sepia-toned prints, gold toners produce red tones. Kodak published formulas for several types of gold toners, which use gold as the chloride.[54] · As gold is a good reflector of electromagnetic radiation such as infrared and visible light as well as radio waves, it is used for the protective coatings on many artificial satellites, in infrared protective faceplates in thermal protection suits and astronauts' helmets and in electronic warfare planes like the EA-6B Prowler. · Gold is used as the reflective layer on some high-end CDs. · Automobiles may use gold for heat shielding. McLaren uses gold foil in the engine compartment of its F1 model.[55] · Gold can be manufactured so thin that it appears transparent. It is used in some aircraft cockpit windows for de-icing or anti-icing by passing electricity through it. The heat produced by the resistance of the gold is enough to deter ice from forming.[56] Electronics The concentration of free electrons in gold metal is 5.90×1022 cm−3. Gold is highly conductive to electricity, and has been used for electrical wiring in some high-energy applications (only silver and copper are more conductive per volume, but gold has the advantage of corrosion resistance). For example, gold electrical wires were used during some of the Manhattan Project's atomic experiments, but large high current silver wires were used in the calutron isotope separator magnets in the project. Though gold is attacked by free chlorine, its good conductivity and general resistance to oxidation and corrosion in other environments (including resistance to non-chlorinated acids) has led to its widespread industrial use in the electronic era as a thin layer coating electrical connectors of all kinds, thereby ensuring good connection. For example, gold is used in the connectors of the more expensive electronics cables, such as audio, video and USB cables. The benefit of using gold over other connector metals such as tin in these applications is highly debated. Gold connectors are often criticized by audio-visual experts as unnecessary for most consumers and seen as simply a marketing ploy. However, the use of gold in other applications in electronic sliding contacts in highly humid or corrosive atmospheres, and in use for contacts with a very high failure cost (certain computers, communications equipment, spacecraft, jet aircraft engines) remains very common.[57] Besides sliding electrical contacts, gold is also used in electrical contacts because of its resistance to corrosion, electrical conductivity, ductilityand lack of toxicity.[58] Switch contacts are generally subjected to more intense corrosion stress than are sliding contacts. Fine gold wires are used to connect semiconductor devices to their packages through a process known as wire bonding. Commercial chemistry Gold is attacked by and dissolves in alkaline solutions of potassium or sodium cyanide, to form the salt gold cyanide—a technique that has been used in extracting metallic gold from ores in the cyanide process. Gold cyanide is the electrolyte used in commercial electroplating of gold onto base metals and electroforming. Gold chloride (chloroauric acid) solutions are used to make colloidal gold by reduction with citrate or ascorbate ions. Gold chloride and gold oxide are used to make highly valued cranberry or red-colored glass, which, like colloidal gold suspensions, contains evenly sized spherical gold nanoparticles (see also nanoparticle).[59] Cultural history
The Turin Papyrus Map
Funerary mask ofTutankhamun
Jason returns with the golden fleece on an Apulian red-figurecalyx krater, ca. 340–330 BC. Gold artifacts found at the Nahal Kana cave cemetery dated during the 1980s, showed these to be from within the Chalcolithic, and considered the earliest find from the Levant (Gopher et al. 1990).[60] Gold artifacts in the Balkans also appear from the 4th millennium BC, such as those found in the Varna Necropolis near Lake Varna in Bulgaria, thought by one source (La Niece 2009) to be the earliest "well-dated" find of gold artifacts.[61] Gold artifacts such as the golden hats and the Nebra disk appeared in Central Europe from the 2nd millennium BC Bronze Age. Egyptian hieroglyphs from as early as 2600 BC describe gold, which king Tushratta of the Mitanni claimed was "more plentiful than dirt" in Egypt.[62] Egypt and especially Nubia had the resources to make them major gold-producing areas for much of history. The earliest known map is known as the Turin Papyrus Map and shows the plan of a gold mine in Nubia together with indications of the localgeology. The primitive working methods are described by both Strabo and Diodorus Siculus, and included fire-setting. Large mines were also present across the Red Sea in what is now Saudi Arabia. The legend of the golden fleece may refer to the use of fleeces to trap gold dust from placer deposits in the ancient world. Gold is mentioned frequently in the Old Testament, starting with Genesis 2:11 (at Havilah), the story of The Golden Calf and many parts of the temple including theMenorah and the golden altar. In the New Testament, it is included with the gifts of the magi in the first chapters of Matthew. The Book of Revelation 21:21 describes the city of New Jerusalem as having streets "made of pure gold, clear as crystal". The south-east corner of the Black Sea was famed for its gold. Exploitation is said to date from the time of Midas, and this gold was important in the establishment of what is probably the world's earliest coinage in Lydia around 610 BC.[63] From the 6th or 5th century BC, the Chu (state) circulated the Ying Yuan, one kind of square gold coin. In Roman metallurgy, new methods for extracting gold on a large scale were developed by introducing hydraulic mining methods, especially inHispania from 25 BC onwards and in Dacia from 106 AD onwards. One of their largest mines was at Las Medulas in León (Spain), where seven long aqueducts enabled them to sluice most of a large alluvial deposit. The mines at Roşia Montană in Transylvania were also very large, and until very recently, still mined by opencast methods. They also exploited smaller deposits in Britain, such as placer and hard-rock deposits atDolaucothi. The various methods they used are well described by Pliny the Elder in his encyclopedia Naturalis Historia written towards the end of the first century AD. The Mali Empire in Africa was famed throughout the old world for its large amounts of gold. Mansa Musa, ruler of the empire (1312–1337) became famous throughout the old world for his great hajj to Mecca in 1324. When he passed through Cairo in July 1324, he was reportedly accompanied by a camel train that included thousands of people and nearly a hundred camels. He gave away so much gold that it depressed the price in Egypt for over a decade.[64] A contemporary Arab historian remarked: Gold was at a high price in Egypt until they came in that year. The mithqal did not go below 25 dirhams and was generally above, but from that time its value fell and it cheapened in price and has remained cheap till now. The mithqal does not exceed 22 dirhams or less. This has been the state of affairs for about twelve years until this day by reason of the large amount of gold which they brought into Egypt and spent there [...] —Chihab Al-Umari[65] The Portuguese overseas expansion started in 1415 with the taking of Ceuta, to control the gold trade coming across the desert. Although the caravan trade routes were then diverted, the Portuguese continued expansing southwards along the coast and eventually buying the gold directly (or less indirectly) from the Africans in the Gulf of Guinea.[citation needed] The European exploration of the Americas was fueled in no small part by reports of the gold ornaments displayed in great profusion by Native American peoples, especially in Central America, Peru, Ecuador and Colombia. The Aztecs regarded gold as literally the product of the gods, calling it "god excrement" (teocuitlatl in Nahuatl), and after Montezuma was killed, most of this gold was shipped to Spain.[66] However, for theindigenous peoples of North America, gold was considered useless, and they saw much greater value in other minerals, which were directly related to their utility, such as obsidian, flint, and slate.[67] Gold played a role in western culture, as a cause for desire and of corruption, as told in children's fables like Rumplestiltskin, where the peasant's daughter turns hay into gold, in return for giving up her child when she becomes a princess, and stealing the hen that lays golden eggs in Jack and the beanstalk. The top prize at the Olympic games is the gold medal. There is an age-old tradition of biting gold to test its authenticity. Although this is certainly not a professional way of examining gold, the bite test was not to check if the coin was gold (90% gold coins are fairly strong) but to see if the coin was gold plated lead. A lead coin would be very soft and thus teeth marks would result. Fake gold coins were a common problem before 1932 so weighing a coin and also sliding a coin through a "counterfeit detector" slot was common (making a lead coin thicker would add weight thus why slide it through a measured slot). Most establishments (especially US Western saloons) would never accept a gold (or silver) coin of high value before weighing such an item.[citation needed] Gold in antiquity was relatively easy to obtain geologically; however, 75% of all gold ever produced has been extracted since 1910.[68] It has been estimated that all gold ever refined would form a single cube 20 m (66 ft) on a side (equivalent to 8,000 m3).[68] One main goal of the alchemists was to produce gold from other substances, such as lead — presumably by the interaction with a mythical substance called the philosopher's stone. Although they never succeeded in this attempt, the alchemists promoted an interest in what can be done with substances, and this laid a foundation for today's chemistry. Their symbol for gold was the circle with a point at its center (☉), which was also the astrological symbol and the ancient Chinese character for the Sun. For modern creation of artificial gold by neutron capture, see gold synthesis. Golden treasures have been rumored to be found at various locations, following famous tragedies such as the Jewish temple treasures in the Vatican, following the temple's destruction in 70 AD, a gold stash on the Titanic, the Nazi gold train - following world war two.
Ancient Greek golden decorated crown, funerary or marriage material, 370–360 BC. From a grave in Armento,Campania The Dome of the Rock on the Jerusalem temple site is covered with an ultra-thin golden glasure. The Sikh Golden temple - the Harmandir Sahib is the best known building covered with gold. Similarly the Wat Phra Kaew emerald Budha temple in Thailand has ornamental gold statues walls and roofs. European king and queen's crowns were made of gold, and gold was used for the bridal crown, since antiquity[69] Because of its historically high value, much of the gold mined throughout history is still in circulation in one form or another. Occurrence
This 156-troy-ounce (4.9 kg) nugget, known as the Mojave Nugget, was found by an individual prospector in the Southern California Desert using a metal detector. Gold's atomic number of 79 makes it one of the higher atomic number elements which occur naturally. Like all elements with atomic numbers larger thaniron, gold is thought to have been formed from a supernova nucleosynthesis process. Their explosions scattered metal-containing dusts (including heavy elements like gold) into the region of space in which they later condensed into our solar system and the Earth.[70] Because the Earth was molten when it was just formed, almost all of the gold present on Earth sank into the core. Most of the gold that is present today in the Earth's crust and mantle was delivered to Earth by asteroid impacts during the late heavy bombardment.[71] On Earth, gold is found in ores in rock formed from the Precambrian time onward.[61] It most often occurs as a native metal, typically in a metal solid solutionwith silver (i.e. as a gold silver alloy). Such alloys usually have a silver content of 8–10%. Electrum is elemental gold with more than 20% silver. Electrum's color runs from golden-silvery to silvery, dependent upon the silver content. The more silver, the lower the specific gravity. Native gold occurs as very small to microscopic particles embedded in rock, often together with quartz or sulfide minerals such as "Fool's Gold", which is a pyrite.[72] These are called lode deposits. The metal in a native state is also found in the form of free flakes, grains or larger nuggets[61] that have been eroded from rocks and end up in alluvial deposits called placer deposits. Such free gold is always richer at the surface of gold-bearing veins[clarification needed] owing to the oxidation of accompanying minerals followed by weathering, and washing of the dust into streams and rivers, where it collects and can be welded by water action to form nuggets.
Relative sizes of an 860 kg block of gold ore, and the 30 g of gold that can be extracted from it. Toi gold mine, Japan.
Gold left behind after a pyrite cube was oxidized to hematite. Note cubic shape of cavity. Gold sometimes occurs combined with tellurium as the minerals calaverite, krennerite, nagyagite, petzite and sylvanite, and as the rare bismuthide maldonite (Au2Bi) and antimonide aurostibite (AuSb2). Gold also occurs in rare alloys with copper, lead, and mercury: the minerals auricupride (Cu3Au), novodneprite (AuPb3) and weishanite ((Au, Ag)3Hg2). Recent research suggests that microbes can sometimes play an important role in forming gold deposits, transporting and precipitating gold to form grains and nuggets that collect in alluvial deposits.[73] Gold in seawater The world's oceans contain gold. Measured concentrations of gold in the Atlantic and Northeast Pacific are 50–150 fmol/L or 10–30 parts per quadrillion (about 10–30 g/km3). In general, gold concentrations for Atlantic and Pacific samples are the same (~50 fmol/L) but less certain. Mediterranean deep waters contain higher concentrations of gold (100–150 fmol/L) attributed to wind-blown dust and/or rivers. At 10 parts per quadrillion the Earth's oceans would hold 15,000 tonnes of gold.[74] These figures are three orders of magnitude less than reported in the literature prior to 1988, indicating contamination problems with the earlier data. A number of people have claimed to be able to economically recover gold from sea water, but so far they have all been either mistaken or acted in an intentional deception. Prescott Jernegan ran a gold-from-seawater swindle in the United States in the 1890s. A British fraudster ran the same scam in England in the early 1900s.[75] Fritz Haber (the German inventor of the Haber process) did research on the extraction of gold from sea water in an effort to help pay Germany's reparations following World War I.[76] Based on the published values of 2 to 64 ppb of gold in seawater a commercially successful extraction seemed possible. After analysis of 4,000 water samples yielding an average of 0.004 ppb it became clear that the extraction would not be possible and he stopped the project.[77] No commercially viable mechanism for performing gold extraction from sea water has yet been identified. Gold synthesis is not economically viable and is unlikely to become so in the foreseeable future. Date: 2016-01-14; view: 536
|