CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
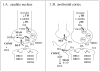
Dopamine systems and their involvement in psychopathologyMost of the dopaminergic neurons in the brain originate from the mesenchephalon and project to other brain regions. These projections are organized into the following three main pathways: nigrostriatal (or, more correctly termed nowadays as mesostriatal, see Björklund and Dunnett, 2007), mesolimbic, and mesocortical pathways. The mesostriatal system projects mainly from the substantia nigra pars compacta to the dorsal striatum (i.e., the caudate nucleus and putamen) and is responsible for motor control; degeneration of this system is responsible for the motor defects in Parkinson s disease (Smith and Villalba, 2008). The mesolimbic system projects from the nucleus paranigralis of the ventral tegmental area (VTA) to the limbic structures, such as the amygdala and the ventral striatum (i.e., the nucleus accumbens and olfactory tubercle). The mesolimbic dopaminergic innervations have an important modulating role in motivated behaviors, which include behavioral activation, exertion of effort in reward-seeking behavior, and maintenance of motivated behavior over time; dysfunctions in this system can contribute to depression, schizophrenia, and drug abuse (Salamone et al., 2005). The mesocortical dopaminergic neurons originate from the nucleus parabrachialis pigmentosus in the VTA and project to the frontal and anterior cingulate cortex, affecting working memory (planning, monitoring, and organizing goal-directed actions based on short-term memory, especially in the face of distractions), cognitive flexibility (the ability to switch task rules), and attentional set shifting (Seamans and Yang, 2004; Robbins and Arnsten, 2009). Within the prefrontal cortex (PFC), there is an optimum concentration of dopamine to perform working memory tasks. This finding is illustrated by the inverted U-shaped response curve showing that an intermediate level of dopamine leads to optimal cognitive performance. This observation is based on animal and human studies demonstrating that excessive dopamine level (acting on D1 receptors) in the PFC leads to impairments in cognitive performance (Robbins and Arnsten, 2009). Dopaminergic axon terminals form inhibitory synapses on the dendritic spines of pyramidal cells in the PFC and exert a gating function on the information flow via the D1 receptors. A very high dopaminergic tone blocks excitatory inputs onto pyramidal cells, whereas too low of a dopaminergic tone facilitates interference between the different inputs. Both of these mechanisms lead to disruptions in cognitive performance. Cortical D2 and D4 receptor functioning has been indicated in cognitive flexibility, attentional set shifting, and decision-making processes (Floresco and Magyar, 2006; Robbins and Arnsten, 2009). In stressful situations, the increased cortical catecholamine (dopamine and norepinephrine) levels impair cognitive functions through network collapse and decreased PFC neuron activity (Arnsten, 2009). Dopamine elimination is different in the PFC compared to other brain areas (Figure 1). Dopamine transporter (DAT) density is the highest in subcortical regions, such as the dorsal striatum, globus pallidus, substantia nigra, and subthalamic nucleus (collectively termed the basal ganglia). In these brain regions DAT is localized at synaptic structures and removes dopamine rapidly from the extracellular space after its release (Figure 1A). In contrast, DAT density is comparatively low in human PFC (Sekine et al., 2001; Tupala et al., 2006), similarly to rat and monkey PFC (Sesack et al., 1998; Lewis et al., 2001). Animal studies also showed that DAT is located at a distance from synaptic sites in the PFC, increasing the probability of substantial extracellular diffusion and dopaminergic transmission at extrasynaptic sites (Sesack et al., 1998, Lewis et al., 2001). Dopamine can be taken up by the norepinephrine transporter in the PFC (Figure 1B), as indicated by animal studies conducted on dopamine and norepinephrine transporter knockout mice (Moron et al., 2002) or by pharmacological evidence with selective norepinephrine transporter blocker atomoxetine (Bymaster et al., 2002). In addition, experiments conducted on catechol-O-methyltransferase (COMT) knockout mice show that this metabolizing enzyme has a major role in dopamine clearence in the PFC (Käenmäki et al., 2010). COMT has a relatively high expression in the human PFC (Matsumoto et al., 2003), although on the protein level it is not well documented (Robinson et al., 1977). However, the observed effects of COMT inhibitor tolcapone on working memory tasks related to PFC function in normal human subjects (Apud et al., 2007; Giakoumaki et al., 2008) supports the importance of COMT in humans as well. It is also important to emphasize the gradually increasing dopaminergic innervation of the PFC during primate development (Wahlstrom et al., 2010), which can be likewise observed in the developmental increase of cortical COMT enzyme activity in humans (Tunbridge et al., 2007). Figure 1 Date: 2016-01-03; view: 755
|