
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Linear Plots Can Be Derived from the Michaelis - Menten Equation
Because of the hyperbolic shape of v versus [S] plots, Vmax can only be determined from an extrapolation of the asymptotic approach of v to some limiting value as [S] increases indefinitely (Figure 14.7); and Km is derived from that value of [S] giving v = Vmax/2. However, several rearrangements of the Michaelis-Menten equation transform it into a straight-line equation. The best known of these is the Lineweaver-Burk double-reciprocal plot:
Taking the reciprocal of both sides of the Michaelis-Menten equation, Equation (14.23), yields the equality
 (14.29)
(14.29)
This conforms to y5mx1b (the equation for a straight line), where y = 1/v; m, the slope, is Km/Vmax; x = 1/[S]; and b = 1/Vmax. Plotting 1/v versus 1/[S] gives a straight line whose x-intercept is -1/ Km, whose y-intercept is 1/ Vmax, and whose slope is Km / Vmax (Figure 14.9).

Figure 14.9 •The Lineweaver-Burk double-reciprocal plot, depicting extrapolations that allow the determination of the x- and y-intercepts and slope.
The Hanes-Woolf plot is another rearrangement of the Michaelis-Menten equation that yields a straight line:
Multiplying both sides of Equation (14.29) by [S] gives
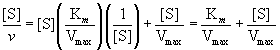 (14.30)
(14.30)
and
 (14.31)
(14.31)
Graphing [S]/v versus [S] yields a straight line where the slope is 1/ Vmax, the y-intercept is Km/Vmax, and the x-intercept is -Km, as shown in Figure 14.10. The common advantage of these plots is that they allow both Km and Vmax to be accurately estimated by extrapolation of straight lines rather than asymptotes. Computer fitting of v versus [S] data to the Michaelis-Menten equation is more commonly done than graphical plotting.
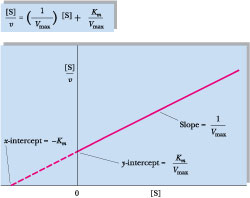 Figure 14.10 • A Hanes-Wolff plot of [S]/v versus [S], another straight-line rearrangement of the Michaelis-Menten equation.
Figure 14.10 • A Hanes-Wolff plot of [S]/v versus [S], another straight-line rearrangement of the Michaelis-Menten equation.
| A Deeper Look | ||||||||||||||||||
| An Example of the Effect of Amino Acid Substitutions on Km and kcat: Wild-Type and Mutant Forms of Human Sulfite Oxidase | ||||||||||||||||||
| Mammalian sulfite oxidase is the last enzyme in the pathway for degradation of sulfur-containing amino acids. Sulfite oxidase (SO) catalyzes the oxidation of sulfite (SO32-) to sulfate (SO42-), using the heme-containing protein, cytochrome c, as electron acceptor: SO32- + 2 cytochrome coxidized + H2O ⇌ SO42- + 2 cytochrome creduced + 2 H+ Isolated sulfite oxidase deficiency is a rare and often fatal genetic disorder in humans. The disease is characterized by severe neurological abnormalities, revealed as convulsions shortly after birth. R. M. Garrett and K. V. Rajagopalan at Duke University Medical Center have isolated the human cDNA for sulfite oxidase from the cells of normal (wild-type) and SO-deficient individuals. Expression of these SO cDNAs in transformed Escherichia coli cells allowed the isolation and kinetic analysis of wild-type and mutant forms of SO, including one (designated R160Q) in which the Arg at position 160 in the polypeptide chain is replaced by Gln. A genetically engineered version of SO (designated R160K) in which Lys replaces Arg160 was also studied. |
Replacing R160 in sulfite oxidase by Q increases Km, decreases kcat, and markedly diminishes the catalytic efficiency (kcat/Km) of the enzyme. The R160K mutant enzyme has properties intermediate between wild-type and the R160Q mutant form. The substrate, SO32-, is strongly anionic, and R160 is one of several Arg residues situated within the SO substrate-binding site. Positively charged side chains in the substrate-binding site facilitate SO32- binding and catalysis, with Arg being optimal in this role. |
Departures from Linearity: A Hint of Regulation?
If the kinetics of the reaction disobey the Michaelis-Menten equation, the violation is revealed by a departure from linearity in these straight-line graphs. We shall see in the next chapter that such deviations from linearity are characteristic of the kinetics of regulatory enzymes known as allosteric enzymes. Such regulatory enzymes are very important in the overall control of metabolic pathways.
Date: 2016-01-03; view: 1107
| <== previous page | | | next page ==> |
| The Michaelis Constant, Km | | | Effect of Temperature on Enzymatic Activity |