
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
What you should be able to do
Make sure you thoroughly understand the following essential ideas which have been presented above.
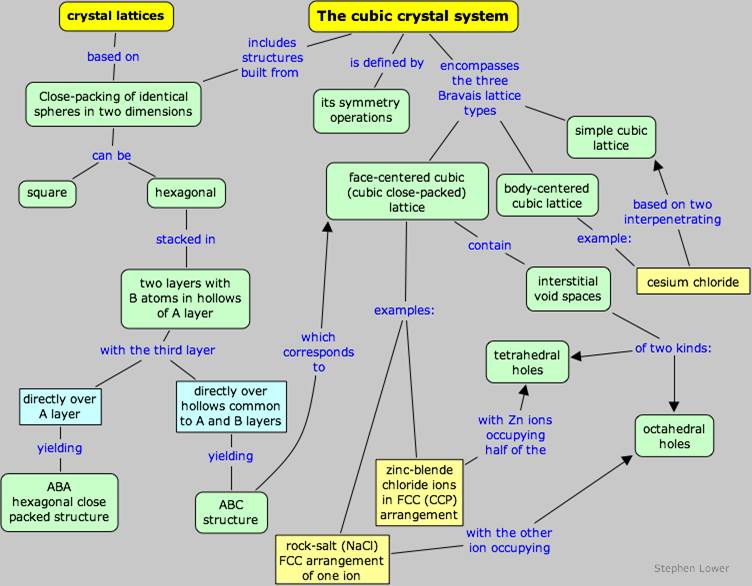
· The difference between square and hexagonal packing in two dimensions.
· The definition and significance of the unit cell.
· Sketch the three Bravais lattices of the cubic system, and calculate the number of atoms contained in each of these unit cells.
· Show how alternative ways of stacking three close-packed layers can lead to the hexagonal or cubic close packed structures.
· Explain the origin and significance of octahedral and tetrahedral holes in stacked close-packed layers, and show how they can arise.
Concept map
Gallery
 BIG halite (NaCl) crystals in the salt mine [Merkers]
BIG halite (NaCl) crystals in the salt mine [Merkers]
|  fluorite (CaF2) [R. Weller]
The colors are due to impurities
fluorite (CaF2) [R. Weller]
The colors are due to impurities
|  sphalerite (ZnS) [theImage]
sphalerite (ZnS) [theImage]
|
More mineral galleries:
Roger Weller, Cochise College - The Mineral Database - Mineral Institute - TheImage

⇐ States of Matter/Solids index ⇐ Virtual Textbook home page
© 2009 by Stephen Lower - last modified 2009-09-01
For information about this Web site or to contact the author,
please see the Chem1 Virtual Textbook home page.

This work is licensed under a Creative Commons Attribution-Share Alike 3.0 License.
Cubic crystal lattices and close-packing
The origins of long-range order in solids
Above: MgO has the same structure as NaCl. [source]
⇐ States of Matter/Solids index ⇐ Virtual Textbook home page
Note: this document will print in an appropriately modified format (12 pages)
On this page:
· Close-packing of identical spheres
· Crystal lattices
· Cubic lattices
· Close-packed lattices in three dimensions
o The two close-packed lattice types
o Hexagonal close-packing
o Cubic close-packing
· Interstitial void spaces
o Tetrahedral holes
o Octahedral holes
· Some common face-centered cubic structures
o The rock-salt structure
o The zinc-blende structure
o The fluorite structure
· Simple- and body-centered cubic structures
o The cesium chloride structure
· What you should be able to do
· Concept map
· Gallery
When substances form solids, they tend to pack together to form ordered arrays of atoms, ions, or molecules that we call crystals. Why does this order arise, and what kinds of arrangements are possible?
These are some of the questions we will explore in this lesson.
We will limit our discussion to cubic crystals, which form the simplest and most symmetric of all the lattice types. Cubic lattices are also very common — they are formed by many metallic crystals, and also by most of the alkali halides, several of which we will study as examples.
Date: 2016-01-03; view: 3208
| <== previous page | | | next page ==> |
| The rock-salt structure | | |