CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Natural vs. synthetic rubberMINISTRY OF EDUCATION AND SCIENCE OF THE REPUBLIC OF KAZAKHSTAN M.AUEZOV SOUTH KAZAKHSTAN STATE UNIVERSITY HIGH SCHOOL “CHEMICAL ENGINEERING AND BIOTECHNOLOGY” CHAIR “PETROLEUM PROCESSING AND PETROCHEMISTRY” REPORT THEME :Production of synthetic rubber Prepared by : Group : Cht-13-6ka2 Checked by : Isa A. Shymkent 2015
Content : Introduction History Application Properties
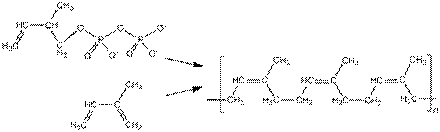
Synthetic rubber, invariably a polymer, is any type of artificial elastomer mainly synthesised from petroleum byproducts. An elastomer is a material with the mechanical (or material) property that it can undergo much moreelastic deformation under stress than most materials and still return to its previous size without permanent deformation. About 15 billion kilograms of rubbers are produced annually, and of that amount two thirds is synthetic. Global revenues generated with synthetic rubbers are likely to rise to approximately US$56 billion in 2020. Synthetic rubber, like natural rubber, has uses in the automotive industry for tires, door and window profiles, hoses, belts, matting, and flooring. Natural vs. synthetic rubber
Chemical structure of cis-polyisoprene, the main constituent of natural rubber. Synthetic cis-polyisoprene and natural cis-polyisoprene are derived from different precursors by different chemical pathways. Natural rubber, coming from latex of Hevea brasiliensis, is mainly poly-cis-isoprene containing traces of impurities like protein, dirt etc. Although it exhibits many excellent properties in terms of mechanical performance, natural rubber is often inferior to certain synthetic rubbers, especially with respect to its thermal stability and its compatibility with petroleum products. Synthetic rubber is made by the polymerization of a variety of petroleum-based precursors called monomers. The most prevalent synthetic rubbers are styrene-butadiene rubbers (SBR) derived from the copolymerization ofstyrene and 1,3-butadiene. Other synthetic rubbers are prepared from isoprene (2-methyl-1,3-butadiene),chloroprene (2-chloro-1,3-butadiene), and isobutylene (methylpropene) with a small percentage of isoprene forcross-linking. These and other monomers can be mixed in various proportions to be copolymerized to produce products with a range of physical, mechanical, and chemical properties. The monomers can be produced pure and the addition of impurities or additives can be controlled by design to give optimal properties. Polymerization of pure monomers can be better controlled to give a desired proportion of cis and trans double bonds. History In 1879, the Frenchman Gustave Bouchardat (1842-1918) created one form of synthetic rubber, producing a polymer of isoprene in a laboratory. The expanded use of motor vehicles, and particularly motor vehicle tires, starting in the 1890s, created increased demand for rubber. In 1909, a team headed by Fritz Hofmann, working at the Bayer laboratory in Elberfeld, Germany, also succeeded in polymerizing methyl isoprene (2,3-dimethyl-1,3-butadiene), the first synthetic rubber. The Russian scientist Sergei Vasiljevich Lebedev created the first rubber polymer synthesized from butadiene in 1910. This form of synthetic rubber provided the basis for the first large-scale commercial production, which occurred during World War I as a result of shortages of natural rubber. This early form of synthetic rubber was again replaced with natural rubber after the war ended, but investigations of synthetic rubber continued. Russian American Ivan Ostromislensky did significant early research on synthetic rubber and a couple of monomers in the early 20th century. Political problems that resulted from great fluctuations in the cost of natural rubber led to the enactment of theStevenson Act in 1921. This act essentially created a cartel which supported rubber prices by regulating production (see OPEC), but insufficient supply, especially due to wartime shortages, also led to a search for alternative forms of synthetic rubber. By 1925 the price of natural rubber had increased to the point that many companies were exploring methods of producing synthetic rubber to compete with natural rubber. In the United States, the investigation focused on different materials than in Europe, building on the early laboratory work of Nieuwland. Studies published in 1930 written independently by Lebedev, the American Wallace Carothers and the German scientist Hermann Staudinger led in 1931 to one of the first successful synthetic rubbers, known as neoprene, which was developed at DuPont under the direction of E.K. Bolton. Neoprene is highly resistant to heat and chemicals such as oil and gasoline, and is used in fuel hoses and as an insulating material in machinery. The company Thiokol applied their name to a competing type of rubber based on ethylene dichloride[5] which was commercially available in 1930. The first rubber plant in Europe SK-1 (from Russian "Synthetic Kauchuk", Russian: ÑÊ-1) was established (Russia) by Sergei Lebedev in Yaroslavl under Joseph Stalin's First Five-Year Plan on July 7, 1932. In 1935, German chemists synthesized the first of a series of synthetic rubbers known as Buna rubbers. These were copolymers, meaning the polymers were made up from two monomers in alternating sequence. Other brands included Koroseal, which Waldo Semon developed in 1935, and Sovprene, which Russian researchers created in 1940. Date: 2015-12-24; view: 1247
|