
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Recovery of Uranium from Simulated Waste Matrices Using Preparative Scale SFE Facility
The results on the extraction and recovery of uranyl ion from various matrices are shown in Table 2. Sc-CO2 modified with methanol containing acetyl acetone was employed for the recovery of uranium. The uranium bearing cellulose matrix was dried at about 50˚C for about 20 minutes in the extraction vessel prior to SFE. Highest recovery of uranyl ion, i.e. ~99% was observed from teflon and glass matrices. The extraction efficiency was found to be ~80% - 83% from cellulose matrix when Sc-CO2 was delivered at flow rate of 30 g/min with a modifier (3 wt% acetyl acetone in methanol) flow rate of 1 mL/min at 200 bar pressure. In these studies, four
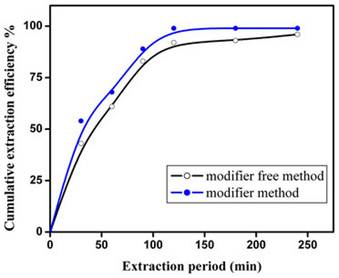
Figure 3. Co-solvent-free delivery of fCMPO for supercritical fluid extraction and recovery of plutonium from cellulose waste matrix. Experimental: Sc-CO2: 3 mL/min; P: 250 bar; Temperature: 45˚C; Extraction vessel capacity: 1 mL; Sample: cellulose waste with plutonium. Co-solvent method: Modifier (5 g fCMPO and 1.8 mL 16N HNO3 in 100 mL methanol) flow rate: 0.15 mL/min; Co-solvent free method: fCMPO (equilibrated with 4N HNO3) delivery ~50 mg/hr.
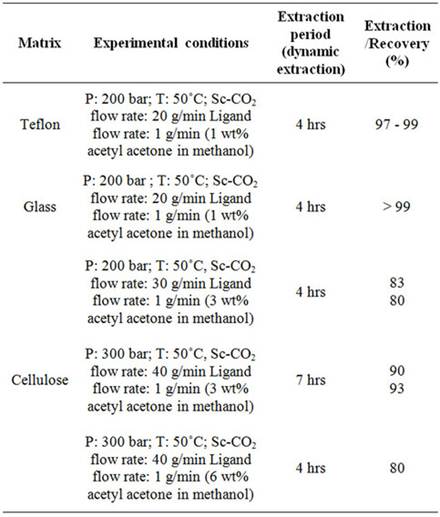
Table 2. Recovery of uranium from simulated waste matrices. In each run, ~2 g uranyl nitrate was loaded on to various matrices.
hours extraction was carried out for the recovery. In another set of experiments, the extraction was carried out at a Sc-CO2 flow rate of 40 g/min for about seven hours; the extraction efficiency was found to be between 90% - 93%. In another experiment, the concentration of acetyl acetone was doubled i.e. 6 wt% acetyl acetone in methanol and extraction was done for 4 hrs duration. In these experiments, extraction efficiency of about 80% - 85% was achieved. The extraction profile for the recovery of uranium from cellulose matrix is shown in Figure 4. These studies have established that Sc-CO2 flow rate and acetyl acetone composition in the methanol can be varied to achieve near complete recovery of uranium from waste matrix. The complete recovery of uranium from teflon and glass matrices (~99%) compared to cellulose matrix has established interaction between uranyl ion with cellulose matrix, leading to some reduction in the extraction efficiency under identical conditions.
Our future endeavors include minimization, recovery and reuse of extractans, which are employed with CO2 for the recovery of actinides from various waste matrices. Our future investigations include use of optimum/minimum pressure and temperature with the preparative scale SFE facility for the recovery of actinides from various matrices.
4. Conclusion
Preparative scale supercritical fluid extraction method was developed and demonstrated for the recovery of uranium from simulated waste matrices. Similarly, complete recovery of plutonium was demonstrated from actual cel-
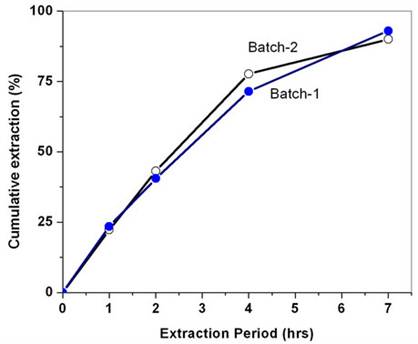
Figure 4. Supercritical fluid extraction and recovery of uranium from simulated cellulose waste matrix. Experimental conditions: P: 300 bar; Temp.: 50˚C; Sc-CO2: 40 g/min; modifier: 3 wt% acetyl acetone in methanol: 1 mL/min. Extraction vessel capacity: 1 litre; Sample: simulated cellulose waste containing uranium.
lulose waste. These studies established that SFE basedtechnique provides an excellent alternative to recover the actinides with minimum generation of secondary liquid waste. The investigations to establish the complete extraction of actinides from waste matrices can lead to various other possible applications in the fuel cycle program.
Date: 2015-01-02; view: 2174
| <== previous page | | | next page ==> |
| Experiments with Preparative Scale SFE System | | | Acknowledgements |