
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
The Next Energy "Game Changer"?
As natural gas from shale becomes a global energy "game changer," oil and gas researchers are working to develop new technologies to produce natural gas from methane hydrate deposits. This research is important because methane hydrate deposits are believed to be a larger hydrocarbon resource than all of the world's oil, natural gas and coal resources combined. [1] If these deposits can be efficiently and economically developed, methane hydrate could become the next energy game changer.
Enormous amounts of methane hydrate have been found beneath Arctic permafrost, beneath Antarctic ice and in sedimentary deposits along continental margins worldwide. In some parts of the world they are much closer to high-population areas than any natural gas field. These nearby deposits might allow countries that currently import natural gas to become self-sufficient. The current challenge is to inventory this resource and find safe, economical ways to develop it.
What is Methane Hydrate?
Methane hydrate is a crystalline solid that consists of a methane molecule surrounded by a cage of interlocking water molecules (see image at top right). Methane hydrate is an "ice" that only occurs naturally in subsurface deposits where temperature and pressure conditions are favorable for its formation. These conditions are illustrated in the phase diagram in the right column of this page.
If the ice is removed from this temperature/pressure environment it becomes unstable. For this reason methane hydrate deposits are difficult to study. They can not be drilled and cored for study like other subsurface materials because as they are brought to the surface the pressure is reduced and the temperature rises. This causes the ice to melt and the methane to escape.
Several other names are commonly used for methane hydrate. These include: methane clathrate, hydromethane, methane ice, fire ice, natural gas hydrate, and gas hydrate. Most methane hydrate deposits also contain small amounts of other hydrocarbon hydrates. These include propane hydrate and ethane hydrate.
Where Are the Methane Hydrate Deposits?
Four Earth environments have the temperature and pressure conditions suitable for the formation and stability of methane hydrate. These are: 1) sediment and sedimentary rock units below Arctic permafrost; 2) sedimentary deposits along continental margins; 3) deep-water sediments of inland lakes and seas; and, 4) under Antarctic ice. [10]. With the exception of the Antarctic deposits, methane hydrate accumulations are not very deep below Earth's surface. In most situations the methane hydrate is within a few hundred meters of the sediment surface.
Deposit models for methane hydrate deposits at continental margins and under permafrost.  In these environments methane hydrate occurs in the sediment as layers, nodules and intergranular cements. The deposits are often so dense and laterally persistent that they create an impermeable layer that traps natural gas moving upwards from below. In 2008, the United States Geological Survey estimated the total undiscovered gas hydrate resource for the Alaska North Slope area. They estimate that the total undiscovered natural gas resource in the form of gas hydrate ranges between 25.2 and 157.8 trillion cubic feet. Because very few wells have been drilled through the gas hydrate accumulations, the estimates have a very high level of uncertainty .
In these environments methane hydrate occurs in the sediment as layers, nodules and intergranular cements. The deposits are often so dense and laterally persistent that they create an impermeable layer that traps natural gas moving upwards from below. In 2008, the United States Geological Survey estimated the total undiscovered gas hydrate resource for the Alaska North Slope area. They estimate that the total undiscovered natural gas resource in the form of gas hydrate ranges between 25.2 and 157.8 trillion cubic feet. Because very few wells have been drilled through the gas hydrate accumulations, the estimates have a very high level of uncertainty .
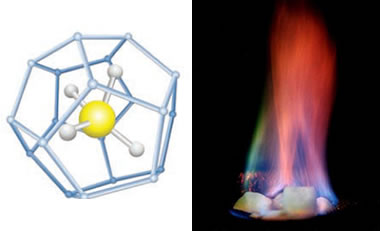
A ball-and-stick model of methane hydrate showing the central methane molecule surrounded by a "cage" of water molecules. Other hydrocarbon molecules such as pentane and ethane can occupy the central position in this structure. (United States Department of Energy image). Right: A burning specimen of methane hydrate ice (United States Geological Survey image). Where is Methane Hydrate Produced Today?
To date there has been no large-scale commercial methane production from gas hydrate deposits. All of the production has either been small scale or experimental.
In early 2012, a joint project between the United States and Japan produced a steady flow of methane by injecting carbon dioxide into the methane hydrate accumulation. The carbon dioxide replaced the methane in the hydrate structure and liberated the methane to flow to the surface. This test was significant because it allowed the production of methane without the instabilities associated with a melting gas hydrate. [6]
The most likely methane hydrate deposits to be selected for first development will have the following characteristics: 1) high concentrations of hydrate; 2) reservoir rocks with high permeability; and, 3) locations where there is an existing infrastructure. [7] Deposits meeting these characteristics will likely be located on the Alaska North Slope or in northern Russia.
Methane Hydrate Hazards
Methane hydrates are sensitive sediments. They can rapidly dissociate with an increase in temperature or a decrease in pressure. This dissociation produces free methane and water. The conversion of a solid sediment into liquids and gases will create a loss of support and shear strength. These can cause submarine slumping, landslides or subsidence that can damage production equipment and pipelines.
Methane is a powerful greenhouse gas. Warmer Arctic temperatures could result in gradual melting of gas hydrates below permafrost. Warming oceans could cause gradual melting of gas hydrates near the sediment-water interface. Although many news reports have presented this as a potential catastrophe, USGS research has determined that gas hydrates are currently contributing to total atmospheric methane and that a catastrophic melting of unstable hydrate deposits is unlikely to send large amounts of methane into the atmosphere.
| Did You Know? Methane hydrate has a very high concentration of methane. If you melt a one cubic meter block of methane hydrate, about 160 cubic meters of gaseous methane will be released. |
Date: 2014-12-29; view: 1305
| <== previous page | | | next page ==> |
| Fluorescent Minerals | | | Methods of Diamond Formation |