
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Methodical guidance for laboratory work ¹2
SEMEY STATE MEDICAL ACADEMY
Methodical recommendation for the laboratory lesson
Specialty:0501301General medicine
Discipline: chemistry
Chair:of chemistry and technology of medicine
Higher base medical education, course– 1
Topic ¹2: Bases of the chemical thermodynamics. Thermo-chemical equation.
Semey 2007
Approved
on the chair meeting from “_____”____________2007.
Protocol ¹ ____
Head of the chair of chemistry and technology of medicine,
prof. __________ Gavrilenko I.V
1.Topic ¹2: Bases of the chemical thermodynamics. Thermo-chemical equation.
2.Aim: to study the main question thermodynamic, which is theoretical base for modem bioenergetics and allow the future doctor to know about bioenergetical balance in human organism.
3.Objectives of teaching:to teach to use the thermodynamically calculations for estimation of calorie content of the food and energy characteristic of the biochemical processes. To teach the student practically define the heat of chemical reactions.
4. Basic questions:
1. Law of conservation of energy - the main law of nature.
2. Thermodynamics and bioenergetics. The main concepts and terms of topic.
3. First law of thermodynamics. Internal energy. Enthalpy.
4.Thermo-chemical. Hess’s law. Thermo-chemical calculations.
4. Fuel value of food .
5. Standard condition and thermochemical (enthalpy of formation)
6. Second law of thermodynamics. Spontaneous and non spontaneous processes . Gibb's energy. Entropy.
7. The united law of the thermodynamics. The criteria and direction of the spontaneous processes. Enthalpy and entropy factors in spontaneous process.
8. Specific of the living organisms as the thermodynamic objects.
Methods of teaching:
The determining initial level knowledge’s of student on chemistry. The conversation and questioning on topic of the lesson. Fulfilling the laboratory work and protection of the report.
Methodical guidance for laboratory work ¹2
Experiment ¹1.Determination of the heat of salt dissolution.
Reagent and facility: calorimeter, cylinder, watch, thermometer, salt, distilled water.
1. Pour into the internal vessel 25 ml of distilled water
2. Dip there a thermometer (not touching the bottom of the vessel)
3. Put 1 gm of a salt sample into the vessel, shut the vessel with the plug and start the timer.
4. Measure the temperature of salt dissolution even minute and stir it carefully to dissolve a salt. Write the thermometer indications into the following table.
5. Make conclusion.
            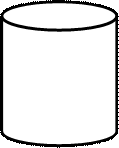  Scheme of calorimeter:
1- internal container
2-heat isolator
3-thermometer
4-mixer Scheme of calorimeter:
1- internal container
2-heat isolator
3-thermometer
4-mixer
| 3 1 |
| time from the beginning of the experiment | ||||||||
| temperature, t, Celcium |
6. t max = t fin, if the reaction is exothermic or
t min = t fin, if the reaction if endothermic.
Calculation
From the table above find temperature change ∆t = t fin -1 initial
Calculate heat of salt dissolution using the following formula

m1-solution weight
m2 - weight of salt
Ñ - specific heat = 4.184 kJ/degree gm
M - molecular mass of salt, gm
Q dissolution= -∆H dissolution
Q (NaNO3)=-21.23kJ/mole


6. Literature:
1. J. M. Sehgal. Modern Chemistry, class XI, Delhi, 1989 - 15 items.
2. Jean B. Umland & John M. Bellama, General Chemistry. Houston. USA. 1996 - 1 item.
3. Darrel D. Ebbing, General Chemistry, Wayne University, USA. 1990 - 3 items.
4. Karen C. Timberlake, Chemistry - An Introduction to General. Organic and Biochemistry, Los Angeles, USA, 1991-1 item.
7. Control:
1. Problems and exercises:
1. At reaction of 2,1 gm of iron with sulphur 30.93 kJ of heat was evolved. Calculate enthalpy of sulphide of iron (II).
2. At reaction of 1,6 gm of bromine with hydrogen (Âr2 + Í2 = 2ÍÂr) 0,72 kJ of heat was evolved. Calculate enthalpy of ÍÂã.
3. Write the mathematical expression of the first effect from law Hess’s for chemical reaction:
à) 2SO2(g) + Î2(g) = 2SO3(g) + DÍ
á) Få2Î3(c) + 3 ÑÎ (g) = 2 Få(c) + 3 ÑÎ2(g) + DÍ
ñ) Ñ6Í12Î6(s) = 2 ÑÎ2 (g) + 2Ñ2Í5ÎÍ(l) + DÍ
4. Calculate heat effect of conversion reactions of the glucose in organism:
Ñ6Í12Î6(c) + 6Î2(g) = 6ÑÎ2 (g) + 6 Í2Î(l) + DÍ
If DÍîáð(glucose) = -1273 kj/mole;
DÍform.(ÑÎ2) = -393,5 kj/mole
DÍform.(Í2Î) = -236 kj/mole
5. Calculate fuel value of glass of milk containing 8 gm of proteins, 4 gm of fat
and 12 gm of carbohydrates. Fuel value of one gram of proteins and
carbohydrates are 4 kcal/gm and fat - 9 kcal/gm.
6. During day student has used in food 80 gm of protein, 105 gm of fat and 450 gm of carbohydrate. Calculate daily calorie content of the food of student, if food value carbohydrates and proteins are 4 kcal/gm, fat - 9 kcal/gm.
7. Calculate at standard conditions heat effect of reactions:
2Ìg (c) + ÑÎ2(g) = 2ÌgÎ(c) + Ñ(c) + DÍ
if DÍform.(ÑÎ2) = -393,5 kj/mole; DÍform.(ÌgÎ) = -602 kj/mole;
8. Without calculations find how the entropy will change in the following
chemical processes:
NÍ4NÎ3(c) = N2Î(c) + 2Í2Î(c)
2Í2 (g) + Î2(g) = 2Í2Î(g)
Í2 (g) + J2 (g) = 2Í2J (g)
NàÑl(ê) +water, solvent= Nà+ (s-n) + Ñl- (s-n)
9. Determine the direction of the spontaneousprocess by energy of Gibbs change:
1) ÑàÑÎ3 (s) = ÑàÎ(s) + ÑÎ2() DG =129 kJ
2) 3 Àl(c) + 3Få3Î4 (c) = 3Få (c) + 4 Àl2Î3 (c) DG =-3285 kJ
3) N2Î4(g) = 2 NÎ2 (g) DG =0 kJ
4) 2ÍJ(g) + Ñl2(g) = 2ÍÑl (g) + J2 (c) DG =-194 kJ
5) N2(g) + 3Í2(g) = 2 NÍ3(g) DG =-3285 kJ
Date: 2015-12-11; view: 3811
| <== previous page | | | next page ==> |
| C. Perpendicular Planes | | | The chemical thermodynamics. Thermo-chemical equation |