
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
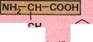
Specialized proteinsHemoglobin is an example of a protein with a quaternary structure. It is the red substance that carries oxygen in the blood. It consists of four polypeptide chains, each of which is grouped around a heme group. Each of the four hemes contains an iron atom that bonds temporarily with oxygen. Like the related compound myoglobin in muscle, hemoglobin is classified as a conjugated protein. Conjugated proteins also contain nonprotein groups. They are important in a number of biological functions. Chlorophyll, the green pigment found in plants, is closely related to heme. But it contains magnesium instead of iron. Antibodies are another type of specialized protein. They form part of the body's defenses against disease. Antibodies bind to a foreign protein or carbohydrate in the blood. This may cause the foreign bodies to clump together (agglutination). Otherwise, the antibodies "tag" the foreign bodies, triggering off a set of processes that results in their destruction. Antibodies combine with a particular point on the surface of the foreign body (called the antigen). Proteins in food can be used by the body as a source of energy. The body's own protein is used up only during starvation. Amino acids cannot be made from inorganic materials by humans. They have to be obtained from food, although some so-called nonessential amino acids can be synthesized from other amino acids. Others, called essential amino acids, cannot be synthesized. A deficiency in the diet will retard growth. Proteincan have great structural strength. It can be formed rapidly, as in the keratin of the antlers of a red deer. Antlers are shed annually and have to be grown afresh every year. Naturally occurring amino acidsfall into two categories. Those such as arginine are called essential amino acids (pink box below). These must be absorbed from food because the body cannot synthesize them. Other amino acids such as alanine are called nonessential (green box below). The adult body can synthesize these amino acids.
|1MH2- CH~COOH| ■f (CH,),NHC(NH)NH, Arginine
I2-|--------- , Histidine N NH |nhj-ch-cooh| CH(CH3)CHZCH3 Isoleucine NH5-CH-COOH -t-CH,CH(CH3)CH, Leucine Lysine
Methionine
r@ Phenylalanine CH(CH3)OH Threonine ■qnoj Tryptophan SN^-H -t-CH(CH3)CH3 Valine
NH5-CH-COOH T CH, Alanine CH2CONH2 Asparagine Aspartic acid Cysteine —1---------- *■ (CH2)2COOH Glutamic acid
—I------------ (CH2)2CONH2 Glutamine Glycine |nh.,-ch-ooh| CH,—CH, \V 2 Proline CH2 Serine NH.T-CH-COOH
r@-QH Tyrosine
Enzymesproduced by yeast play an essential role in brewing. They catalyze (speed up) the fermentation of carbohydrates (sugars and starches) into alcohol (ethanol). Carbon dioxide gas is evolved as a by-product. Substrate links to active site <x> <x>
i t
Active site distorted by alien substrate at secondary site Enzymes
Enzymes are highly specialized proteins that control the chemical reactions in all living cells. They operate as organic catalysts, lowering the amount of energy needed to power a reaction, thus speeding it up. Indeed, many biochemical reactions need large amounts of energy. In the absence of a catalyzing enzyme, most metabolic processes would proceed far too slowly to maintain life. Most enzymes are found within cells. Some are released to catalyze reactions outside cells, such as the digestion of food in the stomach and intestines. Date: 2015-12-11; view: 1248
|

 (CH2)2SCH3
(CH2)2SCH3

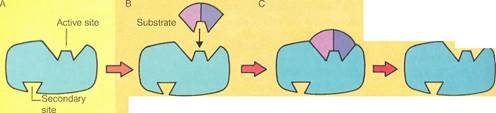 The "lock-and-key" hypothesisis one of the simplest theories of enzyme action. The details of this theory are illustrated on the right. According to the theory, enzymes have an active site called the "lock." Many also have a secondary site (A). The active site is shaped so that only a molecule with the correct complementary shape called the "key" can link to it IB). This forms an enzyme-substrate complex (C). After the reaction has occurred, the products separate from the enzyme (D). The lock-and-key hypothesis also explains how enzyme action may be inhibited. An alien substrate with a shape similar to that of the proper substrate can block the active site (E). Or a different alien substrate can link to the enzyme's secondary site and distort the active site IF).
The "lock-and-key" hypothesisis one of the simplest theories of enzyme action. The details of this theory are illustrated on the right. According to the theory, enzymes have an active site called the "lock." Many also have a secondary site (A). The active site is shaped so that only a molecule with the correct complementary shape called the "key" can link to it IB). This forms an enzyme-substrate complex (C). After the reaction has occurred, the products separate from the enzyme (D). The lock-and-key hypothesis also explains how enzyme action may be inhibited. An alien substrate with a shape similar to that of the proper substrate can block the active site (E). Or a different alien substrate can link to the enzyme's secondary site and distort the active site IF).
