
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Reactions of unsaturated compounds
In general, multiple bonds are more reactive (chemically active) than are single carbon-carbon bonds. Compounds containing multiple bonds may undergo polymerization, cycliza-tion, and addition reactions. Polymerization is the most important of these. Hydrocarbon polymers (large, chainlike molecules) include plastics, resins, and both natural and synthetic rubbers. In a cyclization reaction, a straight chain curls around to form a ring. Several important solvents are made in this way. Addition reactions break one of the bonds in a multiple bond and add two new groups onto the molecule, one at each end of the broken bond. It is this reactivity of the multiple bond, as well as the many synthetic possibilities, that makes these compounds so important in the chemical industry.
One consequence of multiple bonding is that the two linked carbon atoms are not able to rotate freely, as they would if joined by a

|
Car tirescontain a large proportion—more than 25 per cent (by weight) in some cases—of carbon black. Carbon black greatly increases a tire's elasticity, strength, and resistance to wear. The carbon black is produced by burning ethyne in a limited supply of air.
 Organic chemistry: Unsaturated aliphatic hydrocarbons 73
Organic chemistry: Unsaturated aliphatic hydrocarbons 73
|
| Ethyne (acetylene | C8H2) | Propyne (C3H„) | 1-butyne(C1H,.i | 2-butyne (C4H6) | ||||
| H \ | H\ | H I | \ | /H | ||||
| H—C=C- | -H | H^c_c~c- H | -H | H-C-H7 | -c—cs=c- I H | -H | H7l> H | —C—C—C—H XH |


|

|
single bond. If an alkene has two different atoms or groups attached to each carbon atom, it can exist in two physically different forms. The form would depend on whether the groups are on the same side of the molecule or on opposite sides.
Uses of alkenes
Simple alkenes are produced from some types of natural gas. Alkenes can also be produced by cracking naphtha, a straw-colored liquid obtained during the refining of crude oil. Cracking is the process used in the petrochemical industry to break big molecules down into smaller ones. Naphtha, a mixture of saturated hydrocarbons, undergoes thermal cracking at 1000-1200° F. (about 540-650° C) in the absence of air. This breaks it down into unsaturated hydrocarbons such as ethene, pro-pene, and butadiene. Although natural gas is primarily methane, some sources (notably in the U.S.) contain ethene, which can be converted into almost pure ethene.
Ethene is a colorless, highly inflammable gas with a sweet odor and taste. It is the single most important organic chemical feedstock. A feedstock is a principal material in chemical processes that produce petroleum products. Ethene is also an important natural product. It is involved in the ripening of fruits. About half of the ethene (ethylene) produced industrially is used to make the plastic polyethylene. Many other plastics, synthetic rubbers, and resins can also be derived from it. Ethene is used in making antifreeze, cosmetics, paints, lacquers, and acetic acid, a major ingredient of vinegar. Ethyne can also be made from ethene.
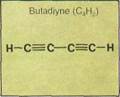
Propene (propylene) is a colorless, inflammable gas that can be used to produce isopro-pyl alcohol, from which acetone is made. Acetone is an important organic solvent for dissolving other substances. Cumene, produced from propene and benzene, is used to make phenol. Phenol (carbolic acid) is used in making antiseptics and disinfectants. Propylene glycol (another alkene product) is a solvent for fats, oils, resins, perfumes, colors and dyes, soft drink syrups, and flavor extracts. Butadiene is important in the manufacture of synthetic rubbers and certain types of resins. These are extremely hard, durable, and resistant to fire.
Higher alkenes can be produced by thermal cracking of waxes, which are saturated hydrocarbons, or by building up a long chain from ethene. They are used to make detergents and lubricants.
Date: 2015-12-11; view: 3278
| <== previous page | | | next page ==> |
| The nylon-making plant | | | Many types of cosmetics, |