
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Production and uses
The first few members of the alkanes, from methane to butane, have important domestic and industrial uses. They are obtained chiefly from fossil fuels, occurring either as underground fields of natural gas or as gas associated with deposits of oil. In some areas, such as under the North Sea, natural gas is almost pure methane. Elsewhere (in the United States, for example) natural gas may contain a significant proportion of other hydrocarbons, such as ethane, propane, and butane. The longer-chained alkanes—gasoline, kerosene, oils, greases, waxes, and bitumen—are obtained by distilling (purifying) petroleum.
Methane is used mainly as a fuel, although it is also important in the manufacture of other
 Organic chemistry: Saturated aliphatic hydrocarbons G9
Organic chemistry: Saturated aliphatic hydrocarbons G9

| H H |
| H H \ / H—C — C —H / \ H H Ethane [CH3CH3i |
| C- I H |
| -C—H H-C- \ / H H |
| -C I H |
| [CR,CH |
H
I
Eh
HH H
Methane [CH4]
The first five straight-chain membersof the al-
kanes are illustrated (above) with their formulas in square brackets. Each member differs from its immediate neighbors by one carbon atom and two hydrogen atoms. This relationship is reflected in the general formula for alkanes: C„H2„+2 (where n is the number of carbon atoms). Only one structure—a straight chain—is possible for the first three alkanes. But with four or more carbon atoms, different, branched-chain structures (which have the
H —C —C-
H H
Propane
[CH3CH?CH3]
same general formula as the straight-chain alkanes) are also possible. The normal forms of butane and pen-tane are known as n-butane and n-pentane. The alternative forms (called isomers) of butane and pentane are illustrated on the right. Butane and pentane have only one and two branched-chain isomers respectively. The number of isomers increases dramatically as the number of carbon atoms increases. A 40-carbon alkane has more than 62 million isomers.
| H | H | H H | H H |
| / | \ | 1 1 | 1 / |
| C-H | H-C- | -C—C- | -C—C-H |
| \ | / | 1 | | 1 \. |
| H y | H [ | H H (-pentane CH3CHZCH | H H 2CH2CH3] |
| \ V /H | H H H H H\ 1 1 / |
| H-C —C —C-H | H —C —C —C—C-H |
| H/ 1 XHC | H" iH NH |
| /|\ | /|\u |
| H H H | H H H |
| Isobutane or | Isopentane or 2-methyibutane |
| 2-methylpropane | [CH3CH(CH3)CH2CH3] |
| [CH3CH(CH3)CH3] | |
| K H H | |
| \ '/ | |
| The branched- | C H I H |
| chain isomers of | \ / |
| butane (above) | H-C — C — C-H H H |
| and pentane (right) | |
| ,0 | |
| /|\ | |
| H i, H | |
| Neopentane or 2,2-dimethyl- | |
| propane [CH3C(CH:1)-CH3] |
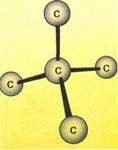
Each carbon atomcan link to four other atoms, including other carbon atoms. When this happens, the atoms tend to arrange themselves so that they are an equal distance apart. This results in the bonds being directed toward the corners of a regular tetrahedron—as illustrated in the diagram above. This diagram shows the structure of diamond, and also the structure of methane (far top left!.
chemicals. Methane can, for example, be converted into methanol and other, longer-chained alcohols, as well as into long-chain alkanes such as gasoline.
The principal use of ethane is as a chemical feedstock, chiefly to make ethene (ethylene). A feedstock is a principal material in chemical processes that produce petroleum products. Ethene can itself be converted into other useful substances—the plastic polyethylene, for example.
Propane and butane can easily be converted into liquids. They are stored in pressurized containers, to be used as a portable fuel supply. They are also used to make other chemicals, such as ethene and propene (propylene).
Syngas
At the present rate of consumption, it is estimated that known reserves of natural gas will last only until about the year 2030. For this reason, there has been considerable research into developing ways of treating coal to make a synthetic gas. This gas, similar to natural gas, is called syngas. It is estimated that reserves of coal will last several hundred years.
When any carbon-containing material is burned in a limited amount of air, carbon monoxide, a poisonous gas, is formed. This gas can be reacted with hydrogen to produce methane. Scientists have developed various ways of making syngas from coal and other organic material. As yet, none is commercially feasible.
Biogas
Methane is also a by-product of the metabolism of some living organisms. In developing countries, such as India and China, farmers build "digesters." A digester is an apparatus that makes methane from waste organic mat-
ter, such as animal dung. In the absence of air, bacteria feed on the waste and give off methane. The local people use the gas for heating and lighting. Such biogas systems are also being investigated in developed countries. They may provide a productive means of disposing of large amounts of waste from the intensive raising of livestock animals.
Date: 2015-12-11; view: 3107
| <== previous page | | | next page ==> |
| Improving on nature | | | Reactions of alkanes |