
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
I I I I I I I I HRHRHRHR
Disproportionation is a rather complicated way in which two growing polymer chains are rendered inactive: when two growing chain ends come close together, the unpaired electron of the chains are exchanged in such a manner that the first chain gains a H element from the second chain, and a double bond forms at the head of the second as delineated below:
Introduction to Polymer Science and Technology Polymerisation
H H HH H(H)H H H H HH H HH
I I I I /TV...............................................
-c-c-c-c *■+ \gfc-c-Cr- -► -c-c-c-CH + c=c-c-c-
I I I I "I I I I I I I I I I I I
HRHR HRHR HRHR HRHR
The addition polymerisation reaction mixture, at a given time, contains monomer, finished polymer, growing polymer chains, and any added reagents.
In the presence of Ziegler-Natta or other stereo-specific catalysts, a growing polymer-chain is attached to the metal atom of the catalyst and subsequent monomer addition is coordinated with the metal atom, known as coordination polymerisation, and leads to highly stereospecific polymerisation. The monomer molecule must sit in a rather specific position in order to react, enabling stereoregular polymers to be made. For example, polymerization of propylene through action of the titanium catalyst gives an isotactic product; whereas, vanadium based catalyst gives a syndiotactic product.
In coordination polymerisation the process is site based and is regulated by the type of active site, whereas in free radical polymerisation the polymerisation site moves progressively away from the initiator and is not influenced by the chemical structure of the initiator, accordingly, it is the type of monomer rather than the initiator that dictates the free-radical polymerisation process. The flexible and dynamic nature of the free-radical site can cause the formation of side branches by an internal chain transfer process known as backbiting, which generates a free radical site within the growing polymer chain. This is much less likely in the coordination polymerisation since no free-radicals are involved in this mechanism, and the active site of the growing chain is at a fixed location on the metal surface of the coordination catalyst.
Introduction to Polymer Science and Technology
Polymerisation
Termination in coordination polymerisation is achieved by reactions of the reactive chain end with an added modifier molecule (a chain transfer agent), e.g., hydrogen, at a concentration of one hydrogen to 1000 ethylene molecules.
| to | H | R | H | R | H | R | H | R | H | R | H | R |
| js. TO | ■-■c- | -C- | -c - | -c - | c- | C-H | + H-H —> H-C- | -c - | -c - | -c - | - c- | - ñ-í |
| î | ||||||||||||
| W | H | H | H | H | H | H | H | H | H | H | H | |
| Growing polymer | chain | Terminated | polymer molecule |
Use of hydrogen provides a low cost clean reaction (no residue) for controlling molecular weight. Temperature affects these processes: higher temperature increases the speed of molecules and causes more collisions, therefore increases reaction rate. However, the reaction of the hydrogen increases even faster, resulting in a higher degree of termination that produces a polymer with a low average molecular weight (high melt-flow index).
2.1.2 Condensation (step-growth) polymerisation
Multifunctional monomers react producing in steps, dimers, trimers, tetramers, oligomers (consisting of 10 to 20 monomer units) and polymer molecules. During the process, links such as ester and amide are formed and a small molecule, e.g., H2O, is eliminated, see Figure 2.1.
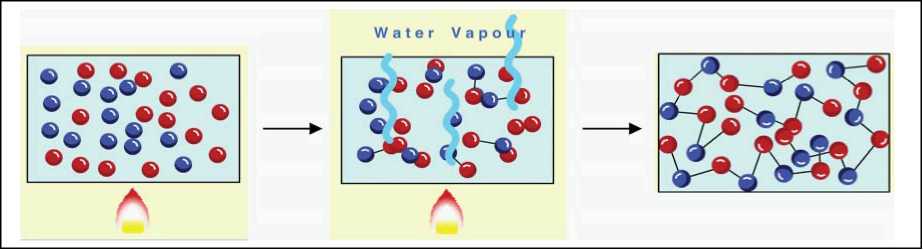
Figure 2.1An illustration of condensation polymerisation
Polyesters contain the ester group, - COO -, which is formed from the reaction between a carboxylic acid and an alcohol. Both these reagents must have two reactive ends (functional groups) in order to enable a chain growth. Poly(ethylene terephthalate) (PET) made from terephthalic acid (benzene-1, 4-dicarboxylic acid) and ethylene glycol (ethane-1,2-diol):
Introduction to Polymer Science and Technology
Polymerisation
| nH2O |
 nHO -CH2 -CH2 -
nHO -CH2 -CH2 -
-[-O-CH2-CH2-O-C-
ester link
Polyamides contain the amide group, -CONH-, a product of reaction between a carboxylic acid and an amine. Both these molecules must have a functionality of two, so that chain growth becomes possible. Nylons are synthetic polyamides made from a variety of dicarboxylic acids and diamines. Usually depending on the number of Ñ atoms in the amine and the acid nylons are designated with numbers, e.g., "Nylon 4,6 or Nylon 46 or PA46" indicates that it is polymerised from a 4-carbon diamine and a 6-carbon diacid. Kevlar is another example, made from terephthalic acid (benzene-1, 4-dicarboxylic acid) and 1, 4-diaminobenzene as shown below.

2.2 Polymerisation processes
The polymerisation process requires a quantity of monomers and a suitable initiator/catalyst system to start the reaction to form polymer molecules that consist of thousands of monomers linked together. Polymerisation processes can be classified in terms of the reaction medium: bulk, solution, suspension, slurry, emulsion and gas.
2.2.1 Bulk polymerisation
Polymerisation of the pure liquid or gaseous monomer is called bulk polymerisation.It can be used for the production of free-radical polymers and some condensation polymers.
In the reaction only monomer, polymer, and initiator are present and, therefore, a very pure product is obtained. The polymerisation is very rapid and strongly exothermic. It can lead to hazardous temperature build up and run away reactions. Overheating can cause branching and crosslinking and lead to the formation of gels The process produces highly transparent polymers: e.g., PS and PMMA.
Date: 2015-12-11; view: 1014
| <== previous page | | | next page ==> |
| Introduction to Polymer Science and Technology Introduction | | | Introduction to Polymer Science and Technology Polymerisation |