
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
ENUMERATION OF BACTERIA
To assess rates of bacterial reproduction, it is necessary to determine numbers of bacteria. Various methods can be employed for enumerating bacteria. These include viable plate count, direct count, and most probable number (MPN) determinations.
Viable Count Procedures
The viable plate countmethod is one of the most common procedures for the enumeration of bacteria. In this procedure, serial dilutions of a suspension of bacteria are plated onto a suitable solid growth
medium and after a period of incubation (during which single cells multiply to form visible colonies) the number of colonies are counted or enumerated (FIG. 10-6).
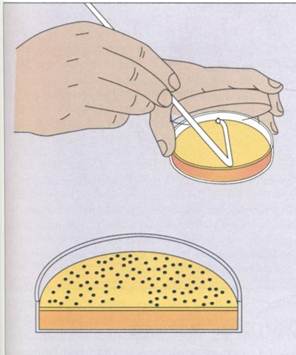
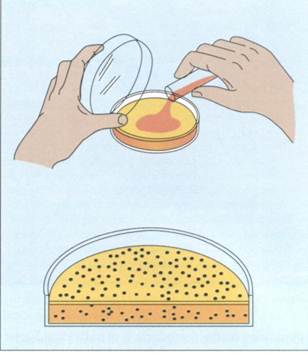
Frequently, the suspension is spread over the surface of an agar plate containing growth nutrients (surface spread technique)(FIG. 10-7). Alternatively, it can be mixed with the agar while it is still in a liqjuid state and poured into the plate (pour plate technique)(FIG. 10-8). The plates are incubated to allow the bacteria to grow and form colonies. The formation of visible colonies generally takes 16 to 24 hours,
|
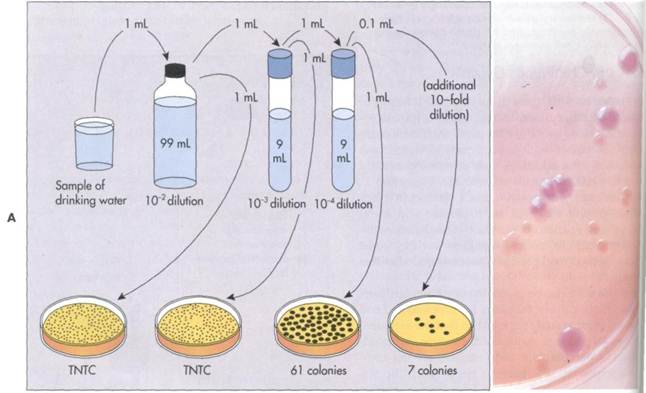
FIG. 10-6A, The plate count procedure is used to determine the viable population in a sample containing bacteria. Dilutions are achieved by adding an aliquot of the specimen to a sterile water dilution tube. If 1 mL of a sample is added to 99 mL of sterile water, the dilution is 1:100 (10~2). (The same dilution could also have been achieved by adding an 0.1 mL sample to 9.9 mL of sterile water). Greater dilutions are achieved by sequentially diluting the sample in series. Adding 1 mL from the first dilution to 9 mL of sterile water achieves an additional tenfold dilution so that the total dilution is 1:1000 (10~3). Adding 1 mL from that second dilution to 9 mL of sterile water achieves a further tenfold dilution so that the total dilution is 1:10000 (10~4). Transferring 1 mL samples from each tube to agar media maintains these dilution factors. Transferring 0.1 mL samples increases the dilution by a factor of 10. After incubation the number of colonies are counted. Counts on the plates in the range of 30 to 300 colonies are used to calculate the concentration of bacteria. The standard notation "TNTC" means too numerous to count (greater than 300 colonies). In this example the plate with 61 colonies would be used to calculate the number of bacteria in the original water sample. Because these colonies developed on a plate in which 1 mL from a 1:10000 dilution was added, the number of bacteria per mL in the original sample is calculated as 6.1 X 105 (61 X 104). B, Colonies of lactose fermenting bacteria growing on MacConkey agar.
ENUMERATION OF BACTERIA 293
|
|

FIG. 10-7The spread plate technique for isolating and enumerating microorganisms.
It is assumed that each colony arises from an individual bacterial cell. By counting the number of colonies that develop, the colony forming units(CFUs), and by taking into account the dilution factors, the concentration of bacteria in the original sample can be determined. Preferably two or three plates are counted to determine numbers of bacteria in a sample. Each plate counted should have 30 to 300
FIG. 10-8The pour plate technique for isolating and enumerating microorganisms.
colonies. If bacterial numbers in a sample are low, it is sometimes necessary to filter the suspension to concentrate the bacterial cells by collecting the cells on a membrane filter. The typical membrane filter for collecting bacterial cells is made of nitrocellulose or cellulose acetate and has a pore size of 0.2 to 0.45 |ëò, which is small enough to trap most bacterial cells. The membrane filter with the trapped bacteria is then placed onto a suitable medium so that bacterial reproduction can occur, and the colonies that develop on the filter are counted. Countable plates are those having between 30 and 300 colonies. Less than 30 colonies is not acceptable for statistical reasons and more than 300 colonies on a plate are likely to produce colonies too close to distinguish as individual CFUs. Such samples are noted as TNTC (too numerous to count).
A major limitation of the viable plate count procedure is that it is selective. There is no single combination of incubation conditions and medium composition that permits the growth of all bacterial types. The nature of the growth medium and the incubation conditions determine which bacteria can grow and thus be counted. Viable counting measures only cells that are capable of growth on the given plating medium under the set of incubation conditions that are used. Sometimes cells are viable but nonculturable unless rigorous steps are taken to acclimate the microorganisms to laboratory culture conditions. The viable plate
Date: 2015-02-28; view: 5711